Patents
Literature
35 results about "NK 1 Receptor Antagonists" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lithium combinations, and uses related thereto
InactiveUS20050233010A1Prevent precipitating manic episodeLessening and preventing riskBiocideNervous disorderPsychoactive drugAdrenergic antagonist
The present invention relates to combinatorial therapies for treating anxiety, depression or psychotic conditions using a lithium salt and a psychoactive drug selected from the group consisting of serotonin reuptake inhibitor, a 5HT2 receptor antagonist, an anticonvulsant, a norepinephrine reuptake inhibitor, an α-adrenoreceptor antagonist, an NK-3 antagonist, an NK-1 receptor antagonist, a PDE4 inhibitor, an Neuropeptide Y5 Receptor Antagonists, a D4 receptor antagonist, a 5HT1A receptor antagonist, a 5HT1D receptor antagonist, a CRF antagonist, a monoamine oxidase inhibitor, a sedative-hypnotic drug, and an atypical antipsychotic.
Owner:NOVEN THERAPEUTICS
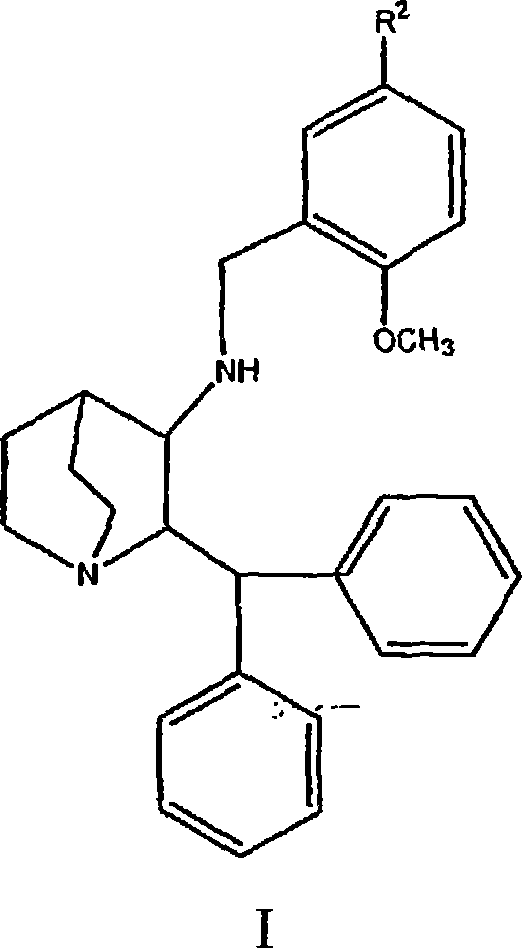
Tachykinin receptor antagonists
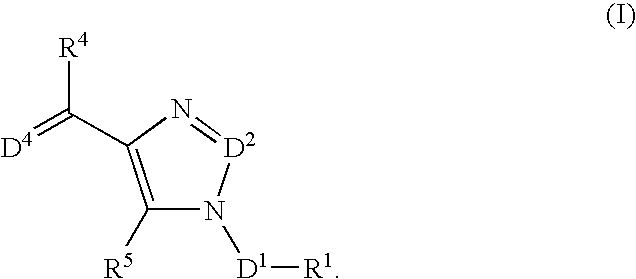
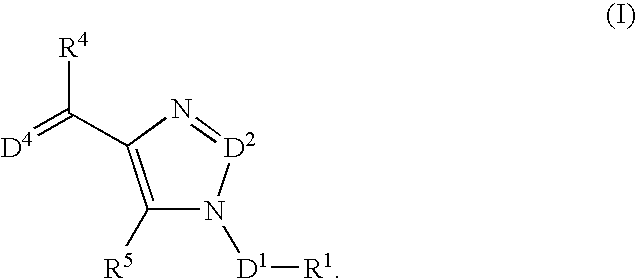
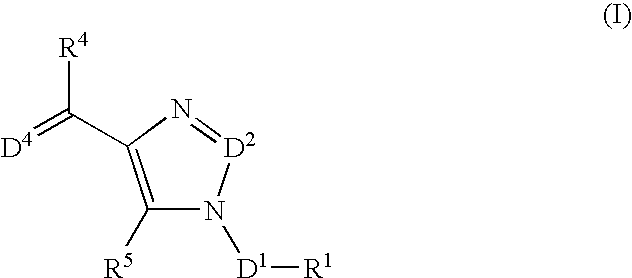
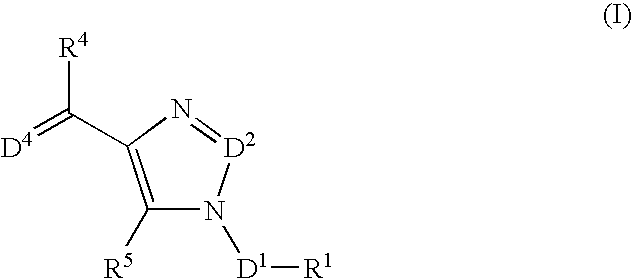
The present invention relates to selective NK-1 receptor antagonists of Formula (I); or a pharmaceutically acceptable salt thereof, for the treatment of disorders associated with an excess of tachykinins
Owner:ELI LILLY & CO
Use of nk-1 receptor antagonists in pruritus
The invention relates to methods for treating pruritus with NK-1 receptor antagonists such as serlopitant. The invention further relates to pharmaceutical compositions comprising NK-1 receptor antagonists such as serlopitant. In addition, the invention encompasses treatment of a pruritus-associated condition with serlopitant and an additional antipruritic agent, and the use of serlopitant as a sleep aid, optionally in combination with an additional sleep-aiding agent.
Owner:VYNE THERAPEUTICS INC
Use of NK-1 receptor antagonists in pruritus
ActiveUS9198898B2Organic active ingredientsOintment deliveryAntipruritic agentsNK 1 Receptor Antagonists
Owner:VYNE THERAPEUTICS INC
Use of NK-1 receptor antagonists in pruritus
The invention relates to methods for treating pruritus with an NK-1 receptor antagonist. The invention further relates to pharmaceutical compositions comprising NK-1 receptor antagonist.
Owner:VYNE THERAPEUTICS INC
Use of nk-1 receptor antagonists in pruritus
The invention relates to methods for treating pruritus with an NK-1 receptor antagonist. The invention further relates to pharmaceutical compositions comprising NK-1 receptor antagonist.
Owner:VYNE THERAPEUTICS INC
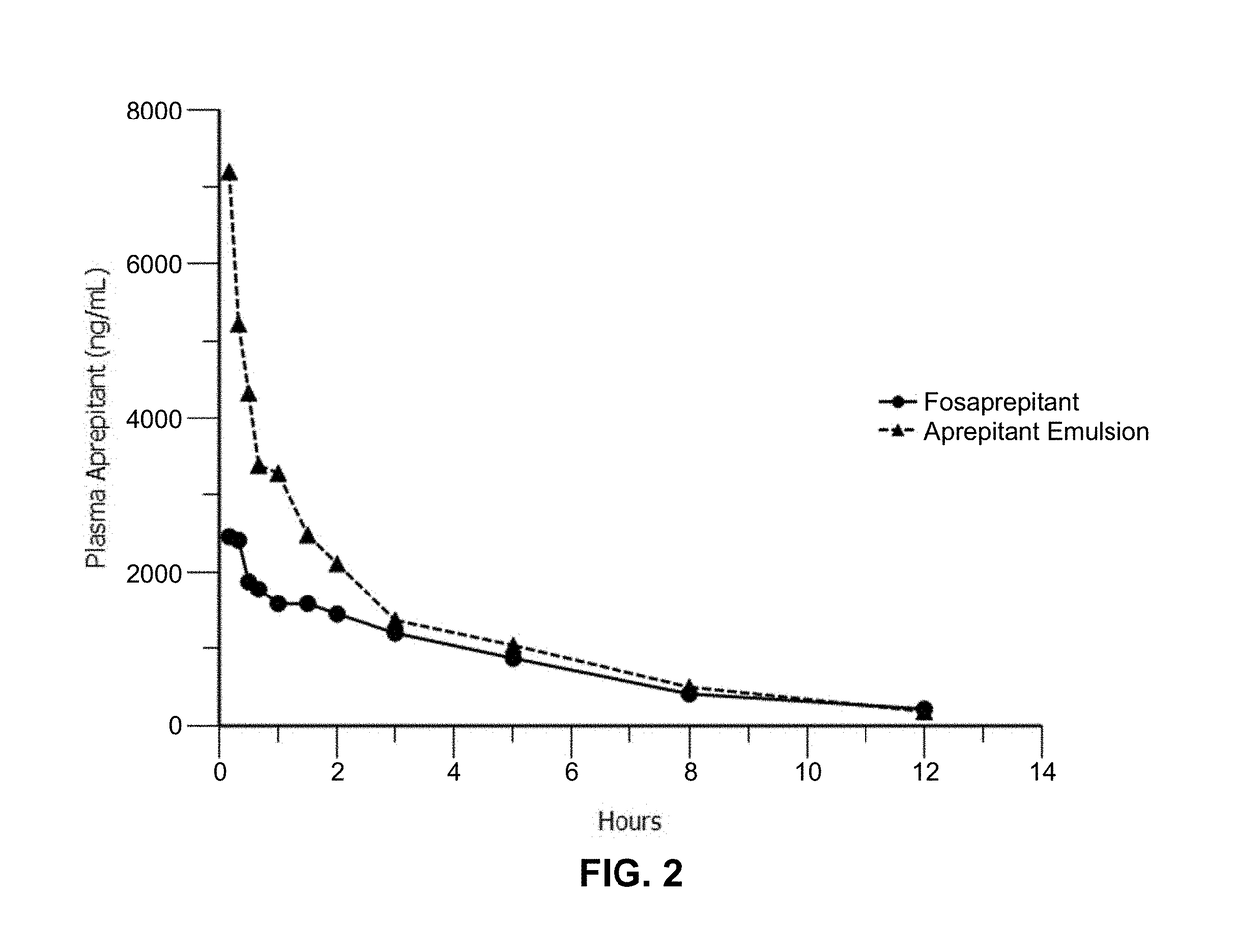
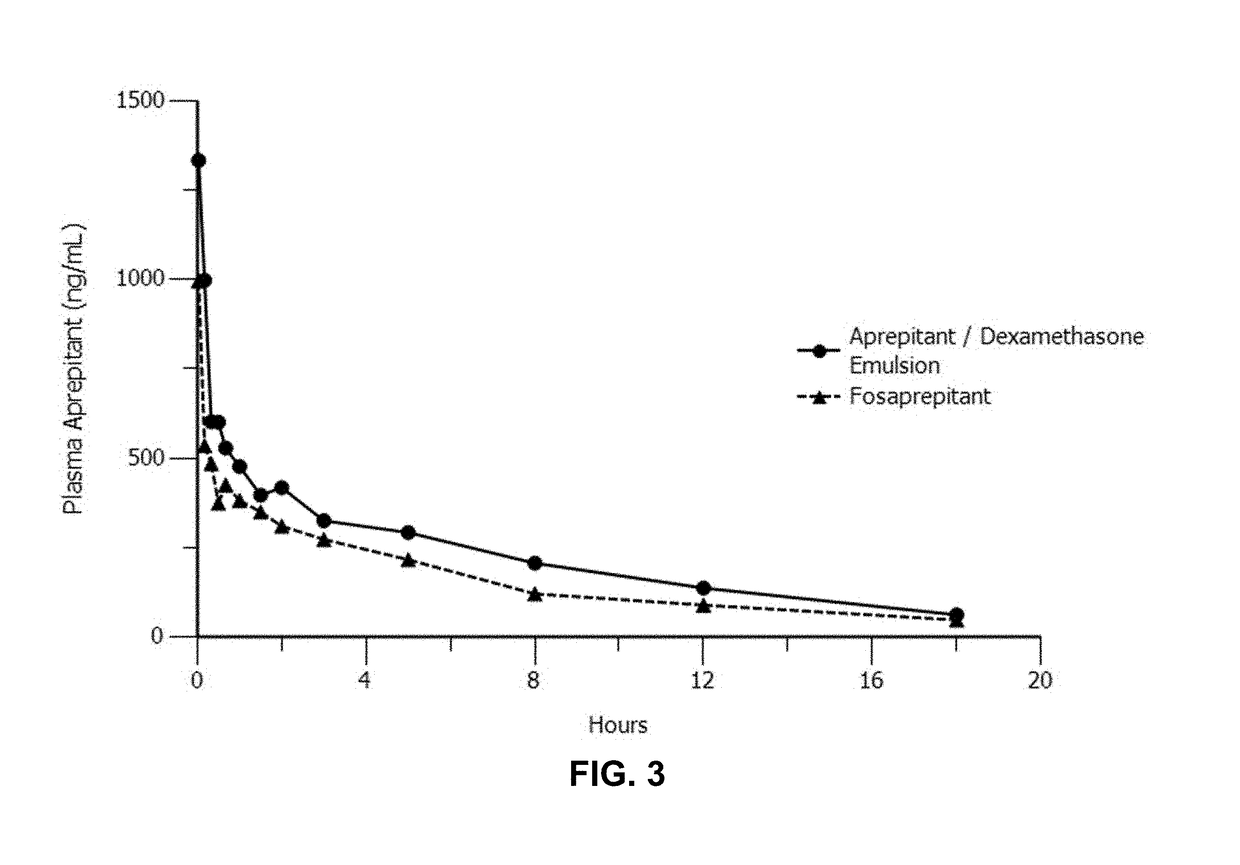
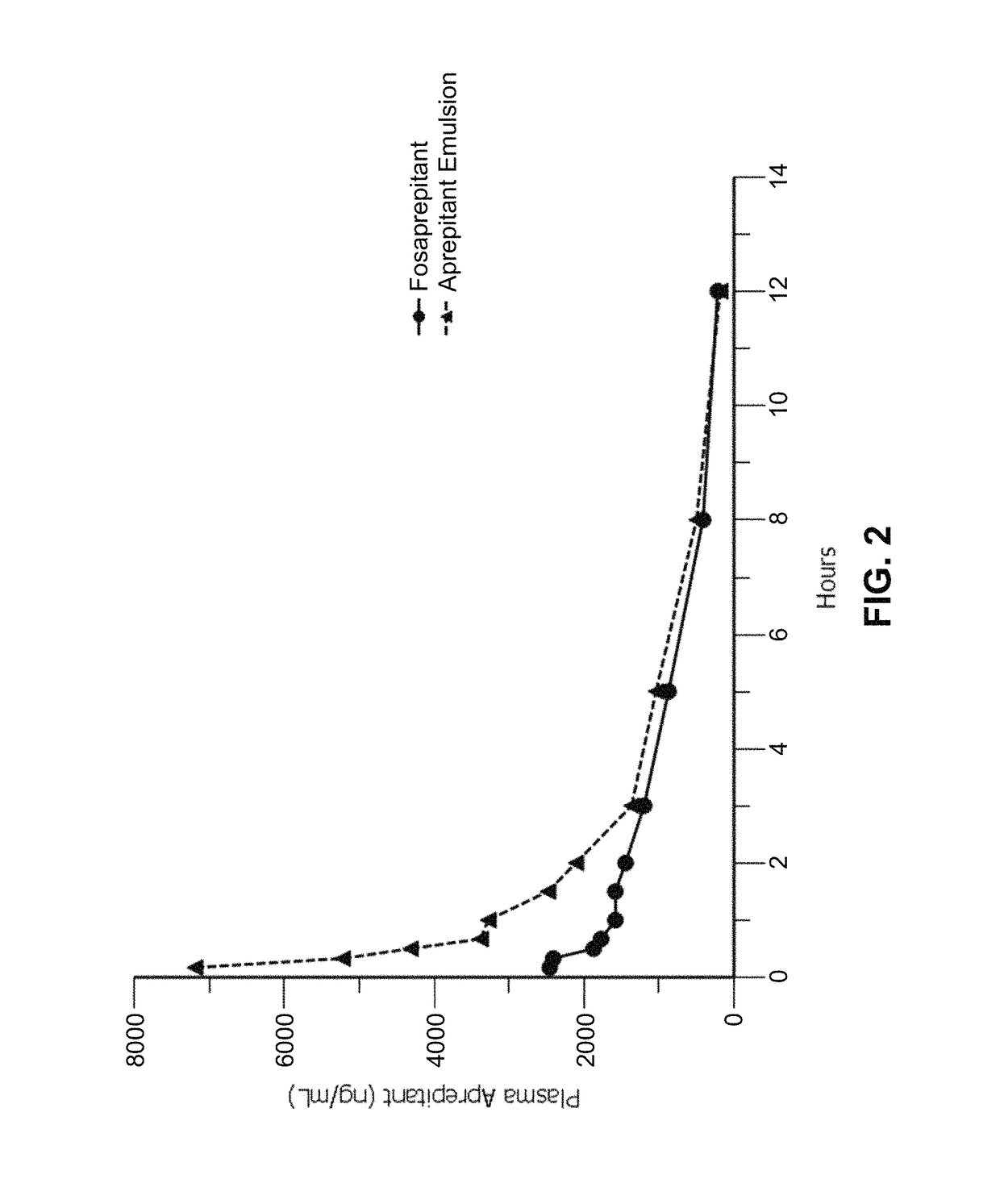
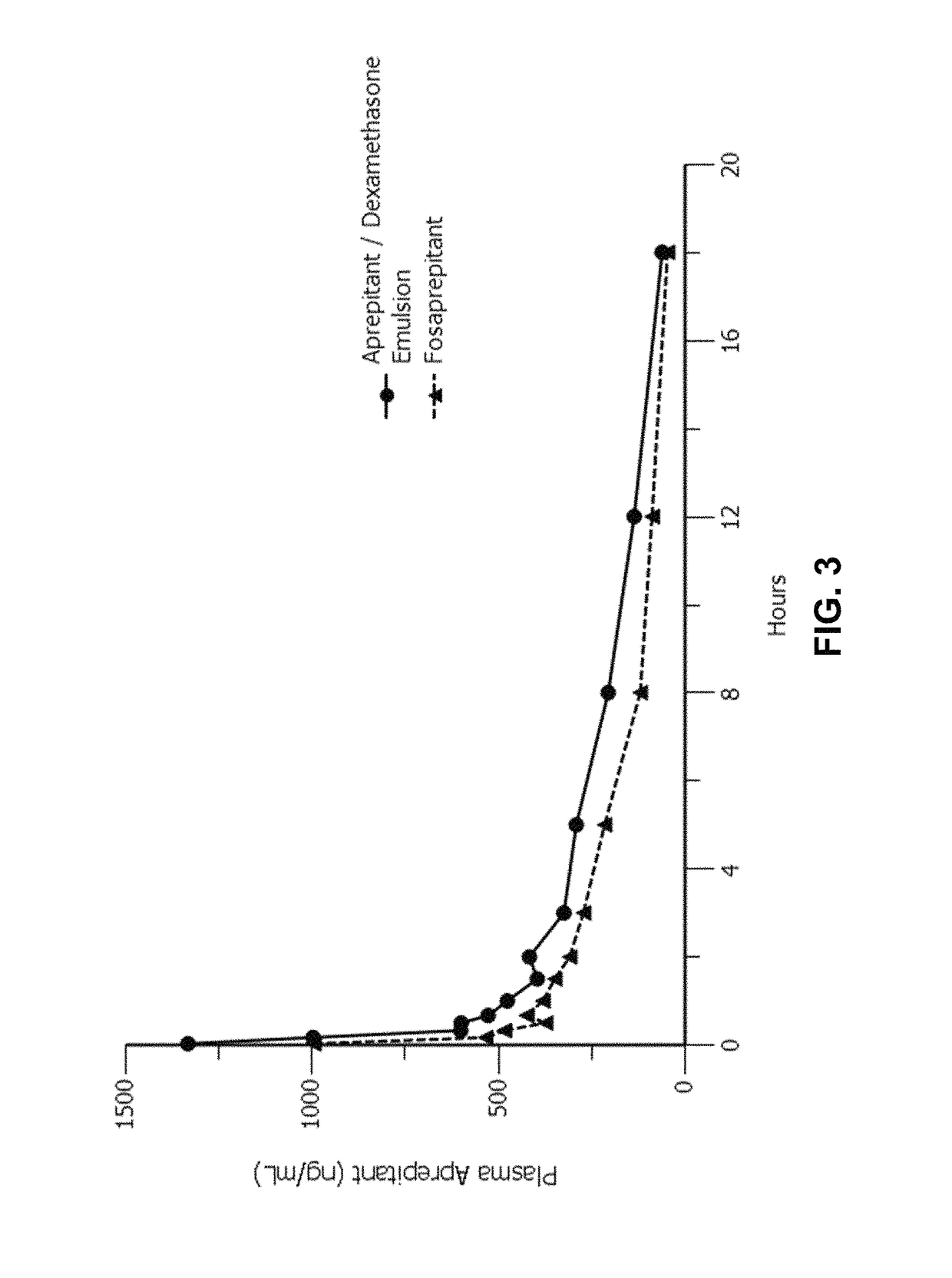
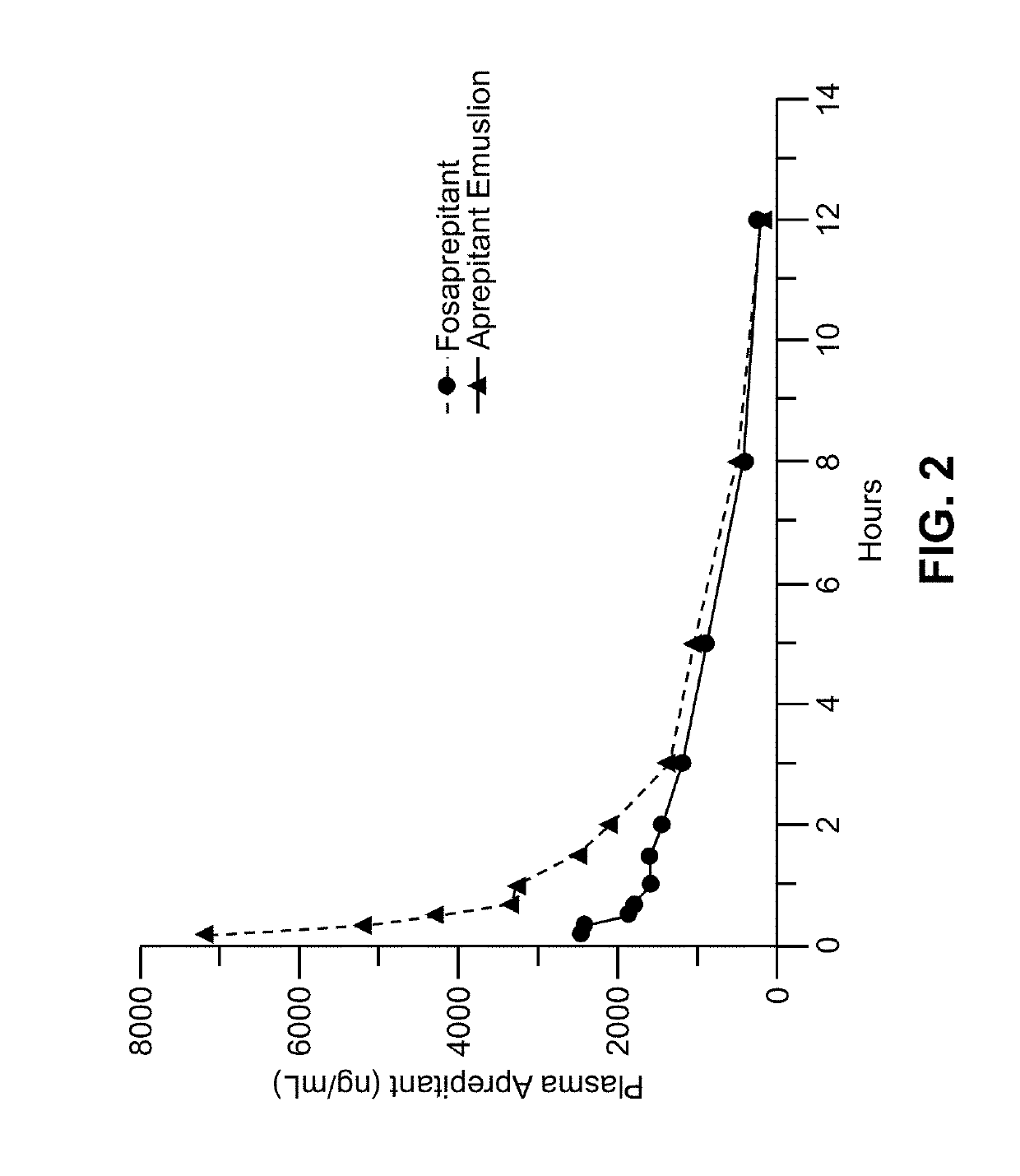
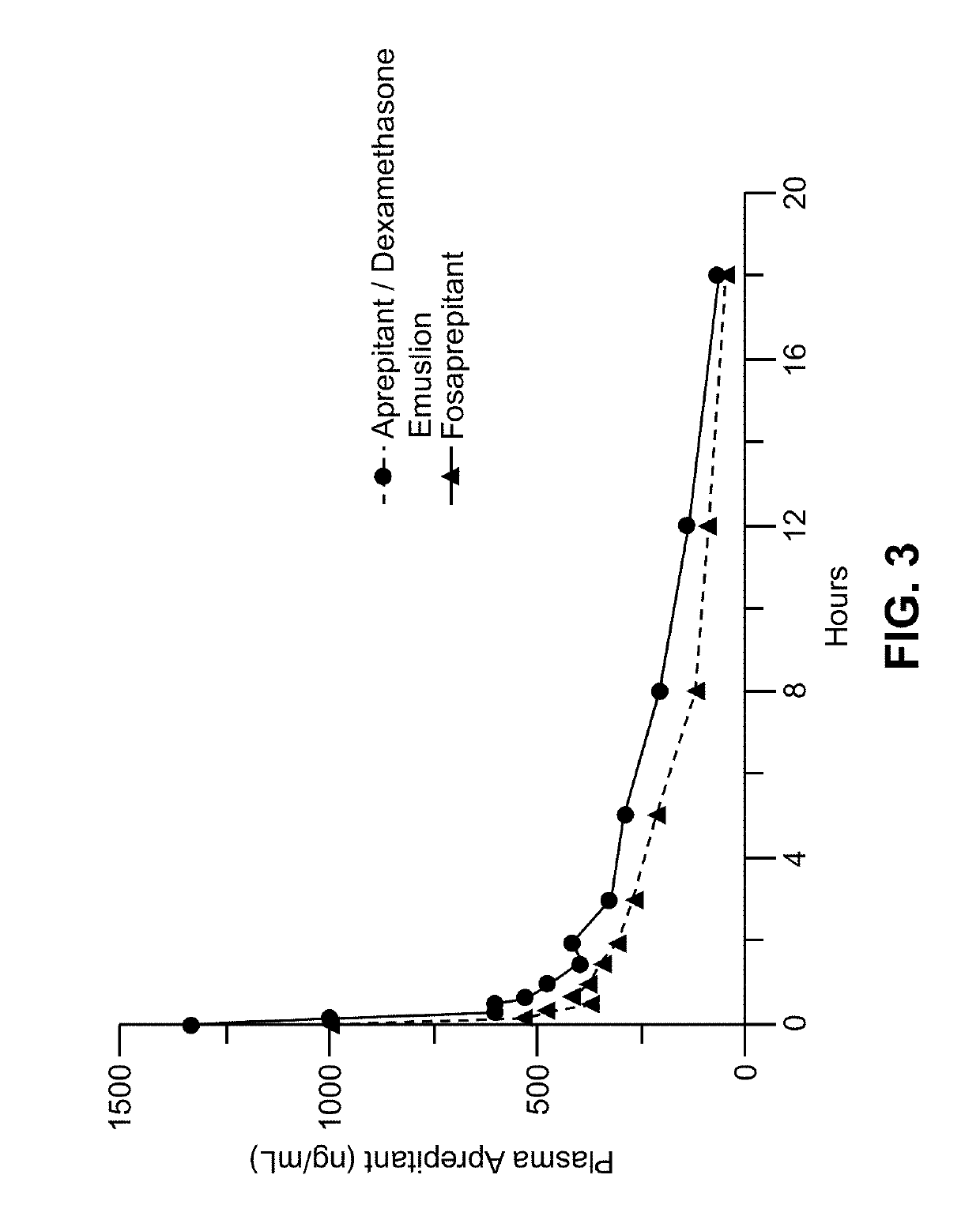
Emulsion formulations of an nk-1 receptor antagonist and uses thereof
Disclosed herein are novel pharmaceutical formulations of a neurokinin-1 (NK-1) receptor antagonist suitable for parenteral administration including intravenous administration. Also included are formulations including both the NK-1 receptor antagonist and dexamethasone sodium phosphate. The pharmaceutical formulations are stable oil-in-water emulsions for non-oral treatment of emesis and are particularly useful for treatment of subjects undergoing highly emetogenic cancer chemotherapy.
Owner:HERON THERAPEUTICS
Use of nk-1 receptor antagonist serlopitant in pruritus
The invention relates to methods for treating pruritus with NK-1 receptor antagonists such as serlopitant. The invention further relates to pharmaceutical compositions comprising NK-1 receptor antagonists such as serlopitant. In addition, the invention encompasses treatment of a pruritus-associated condition with serlopitant and an additional antipruritic agent, and the use of serlopitant as a sleep aid, optionally in combination with an additional sleep-aiding agent.
Owner:VYNE THERAPEUTICS INC
Emulsion formulations of an NK-1 receptor antagonist and uses thereof
Disclosed herein are novel pharmaceutical formulations of a neurokinin-1 (NK-1) receptor antagonist suitable for parenteral administration including intravenous administration. Also included are formulations including both the NK-1 receptor antagonist and dexamethasone sodium phosphate. The pharmaceutical formulations are stable oil-in-water emulsions for non-oral treatment of emesis and are particularly useful for treatment of subjects undergoing highly emetogenic cancer chemotherapy.
Owner:HERON THERAPEUTICS
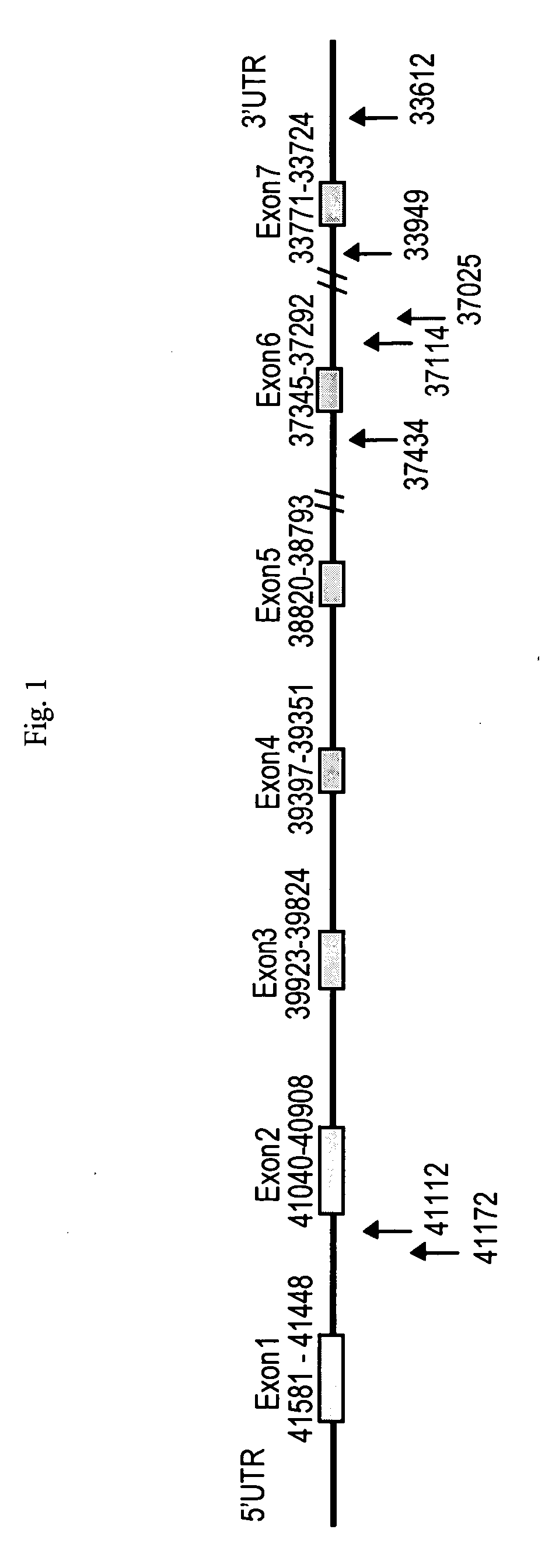
Genetic polymorphisms in the preprotachykinin gene
The present invention relates to a method of correlating single nucleotide polymorphisms in the pre-tachykininogen (NKNA) gene with the efficacy and compatibility of pharmaceutically active compounds administered to human subjects. The present invention also relates to a method of determining the efficacy and compatibility of a pharmaceutically active compound administered to a human individual, the method comprising detecting at least one single nucleotide polymorphism in the NKNA gene. The method is based on the detection of specific single nucleotide polymorphisms in the NKNA gene and the determination of the efficacy and compatibility of pharmaceutically active compounds in human subjects with reference to the NKNA gene polymorphisms. The present invention also relates to isolated nucleic acids comprising polymorphisms as defined herein in their sequence, to nucleic acid primers and oligonucleotide probes capable of hybridizing to such nucleic acids, to nucleic acids comprising one or more of such primers and probes A diagnostic kit for detecting NKNA gene polymorphisms, relating to a pharmaceutical pack containing an NK-1 receptor antagonist and instructions for administering the drug to a human individual subject to polymorphism detection, and also to a stored NKNA gene polymorphism A computer readable medium of state sequence information.
Owner:F HOFFMANN LA ROCHE & CO AG
Use of nk-1 receptor antagonists in management of visceral pain
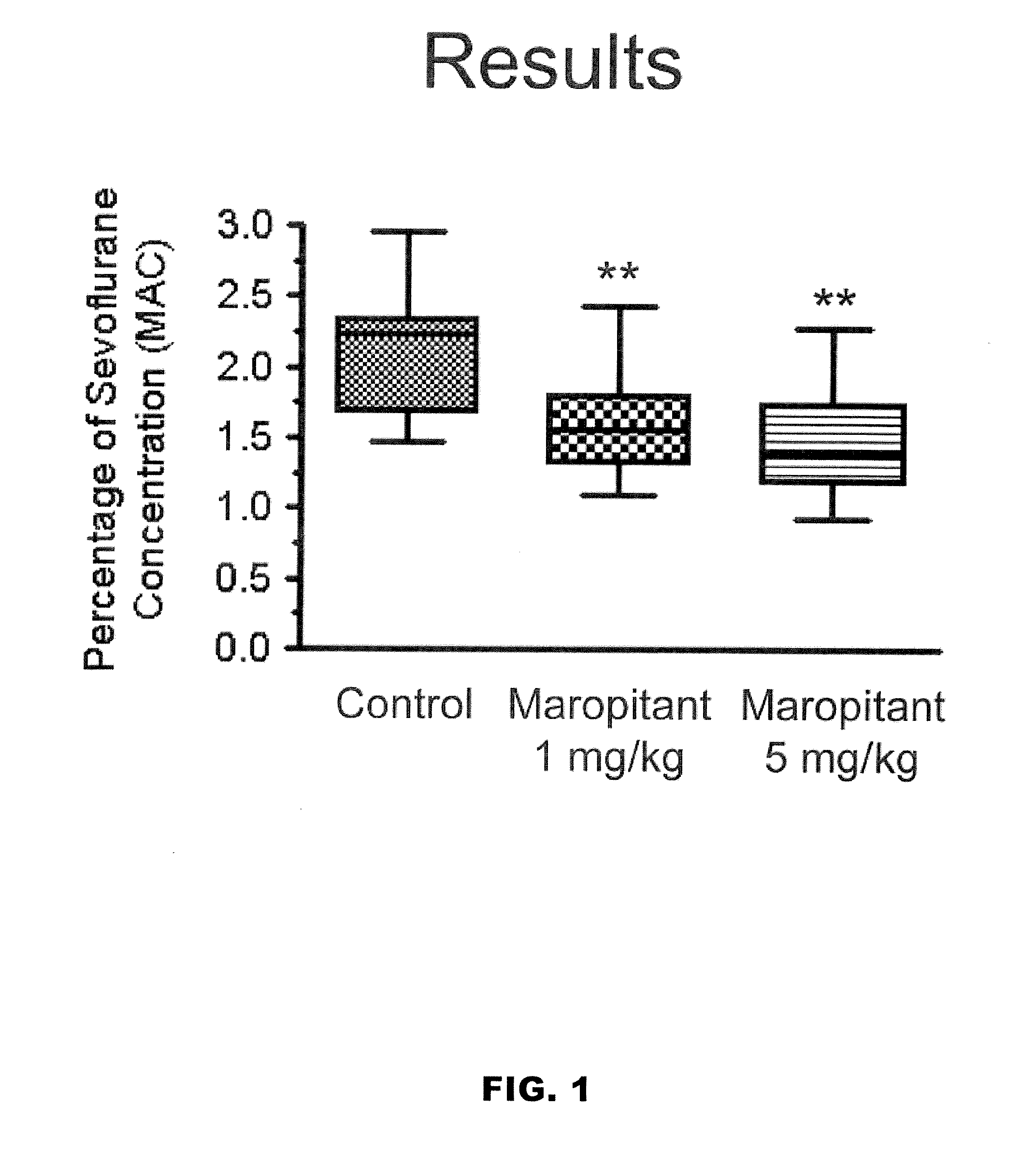
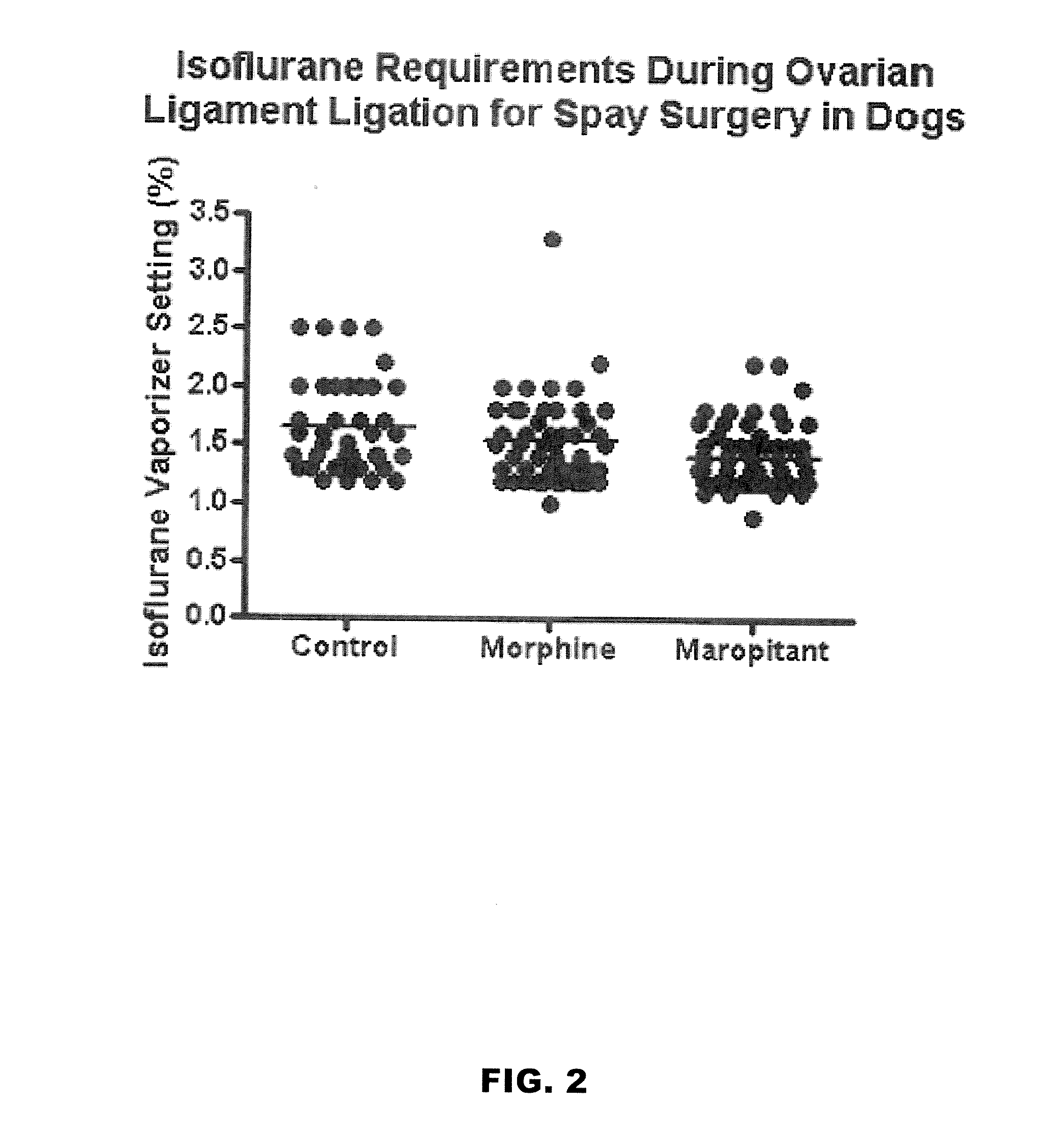
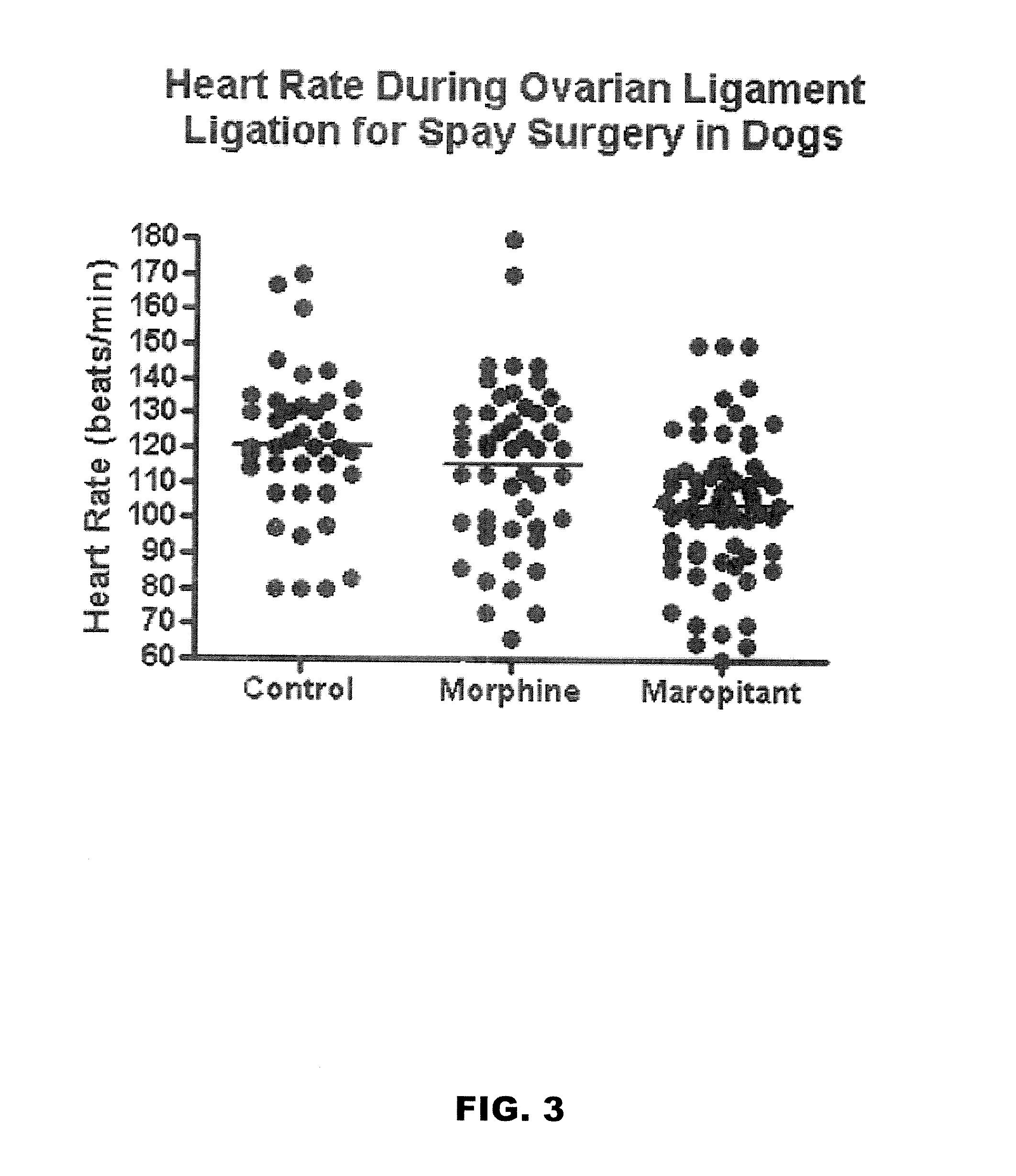
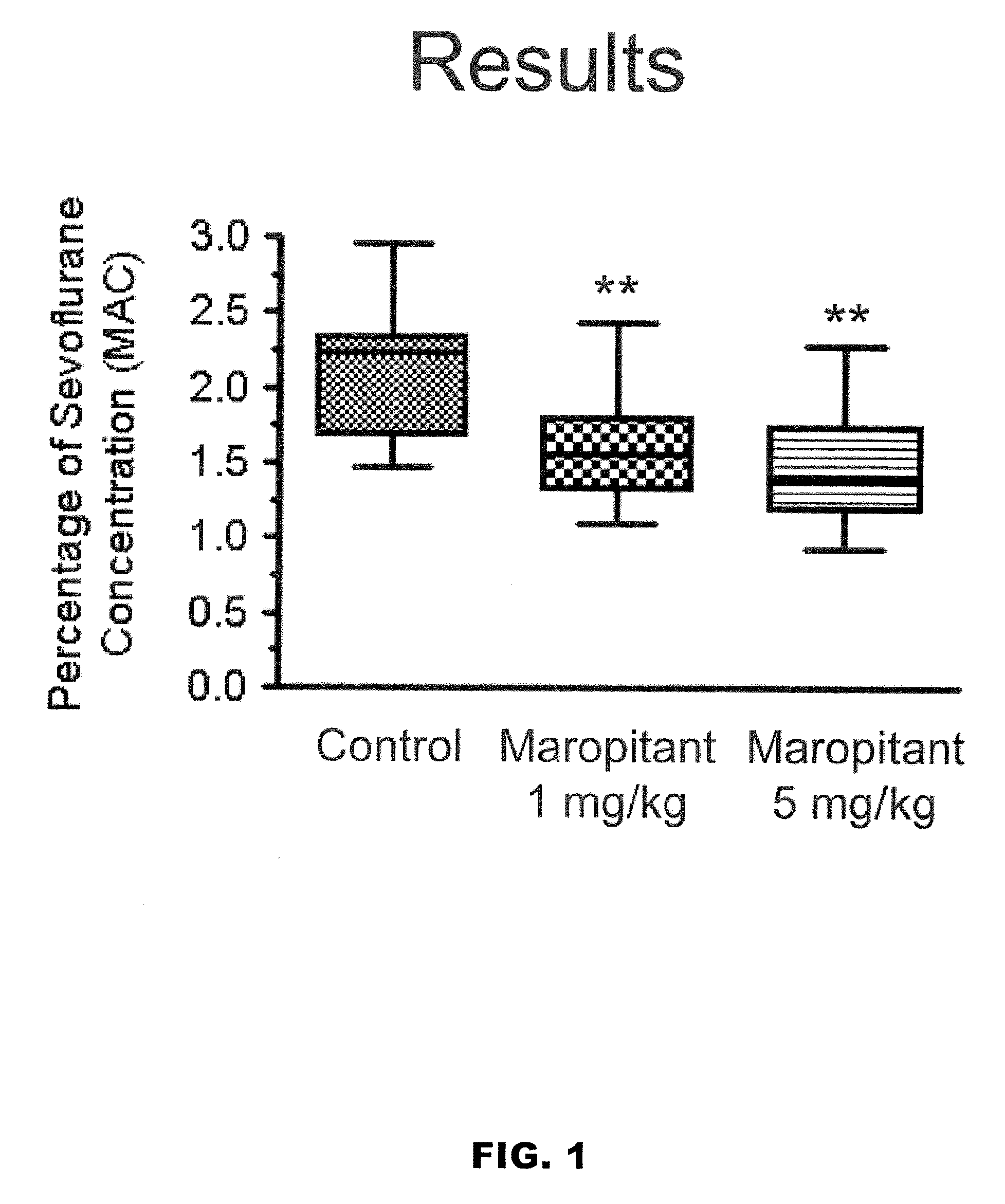
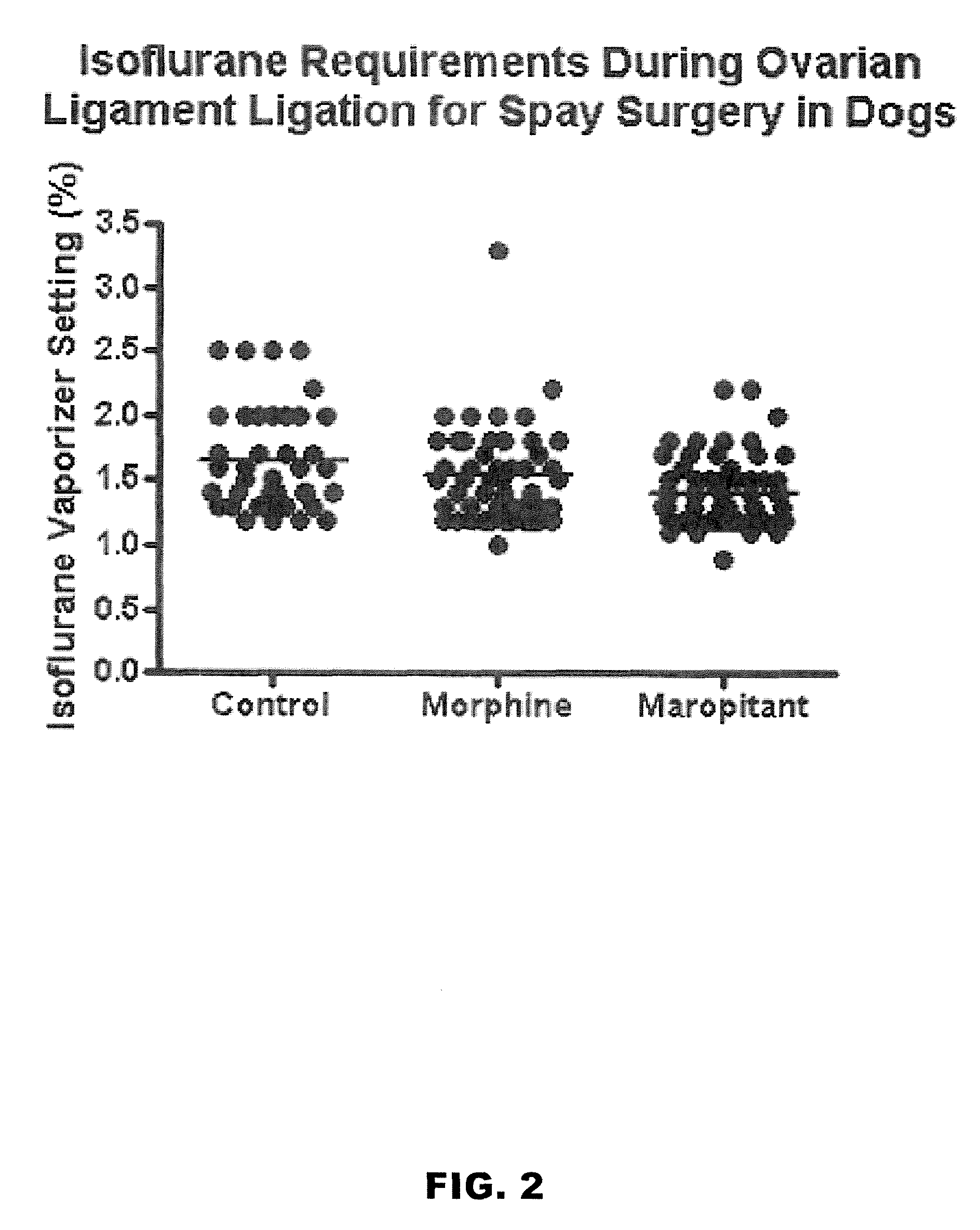
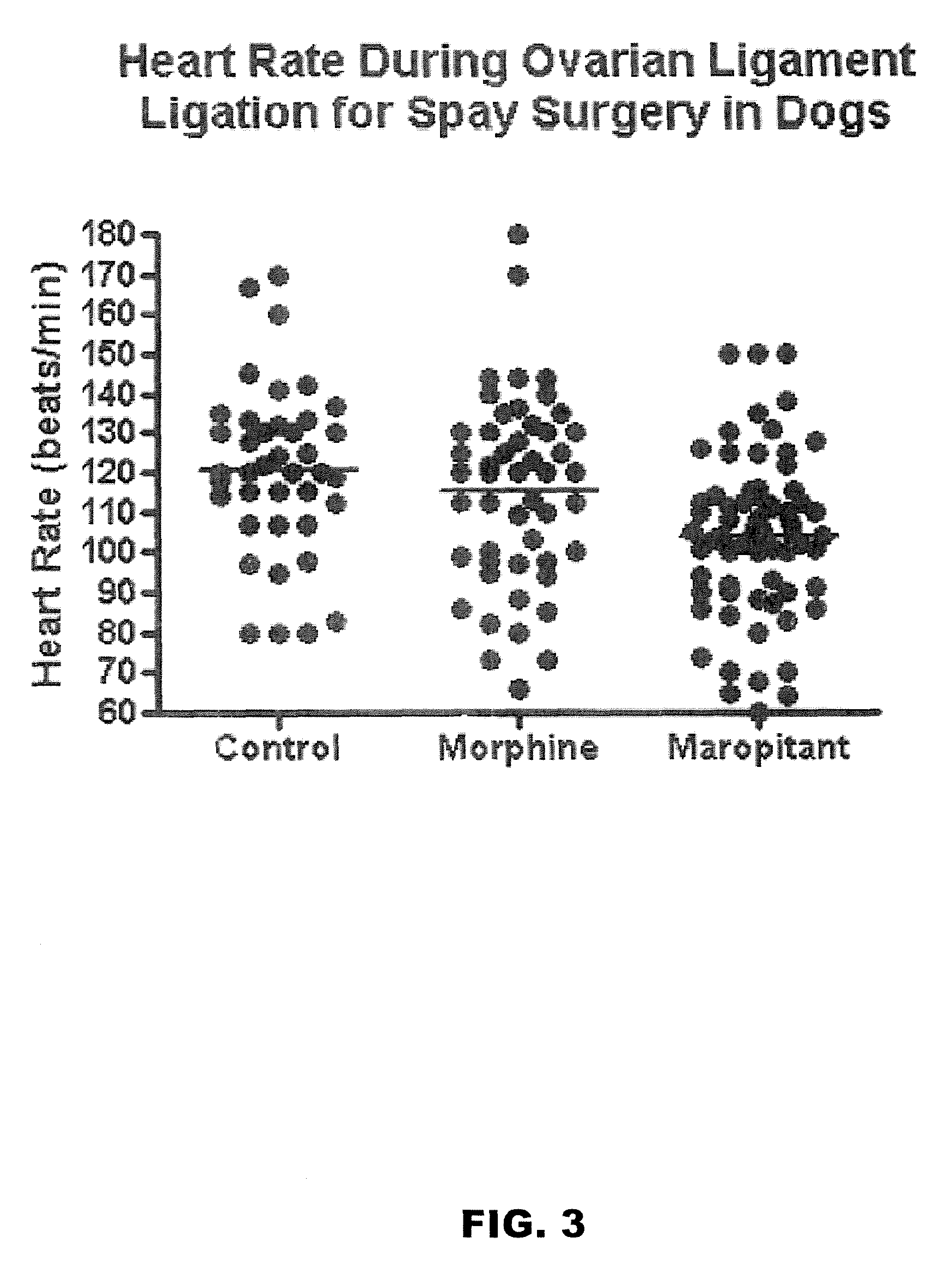
Methods for managing visceral pain in mammalian subjects are described, in which a NK-1 receptor antagonist is administered to the subject before, during or after administration of general anesthesia. The methods and uses of NK-1 receptor antagonists described herein provide improved visceral pain management and MAC reduction when used with volatile anesthetics for general anesthesia.
Owner:COLORADO STATE UNIVERSITY
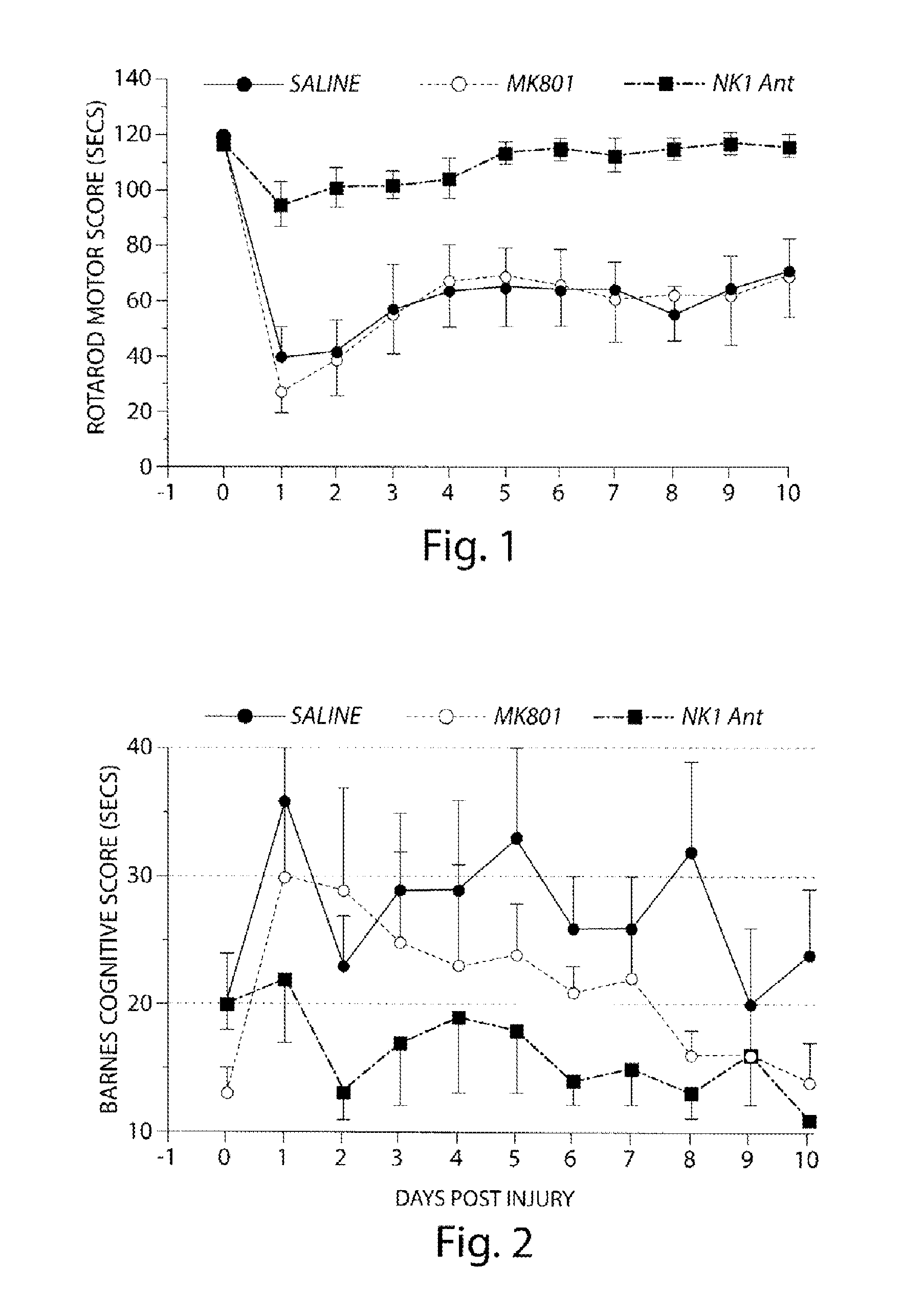
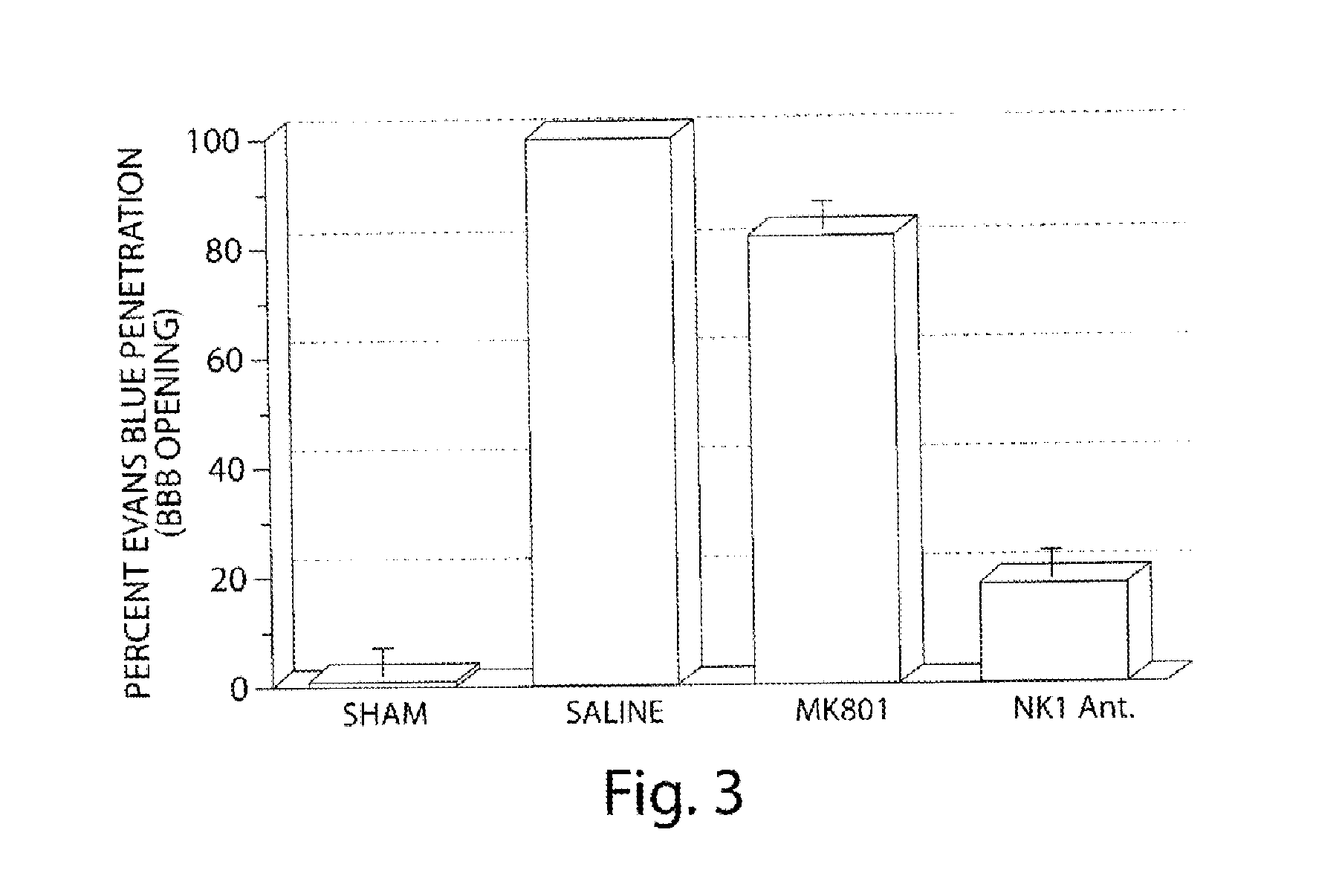
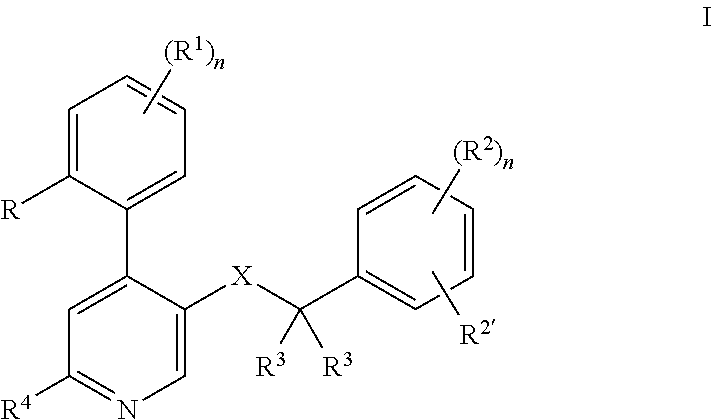
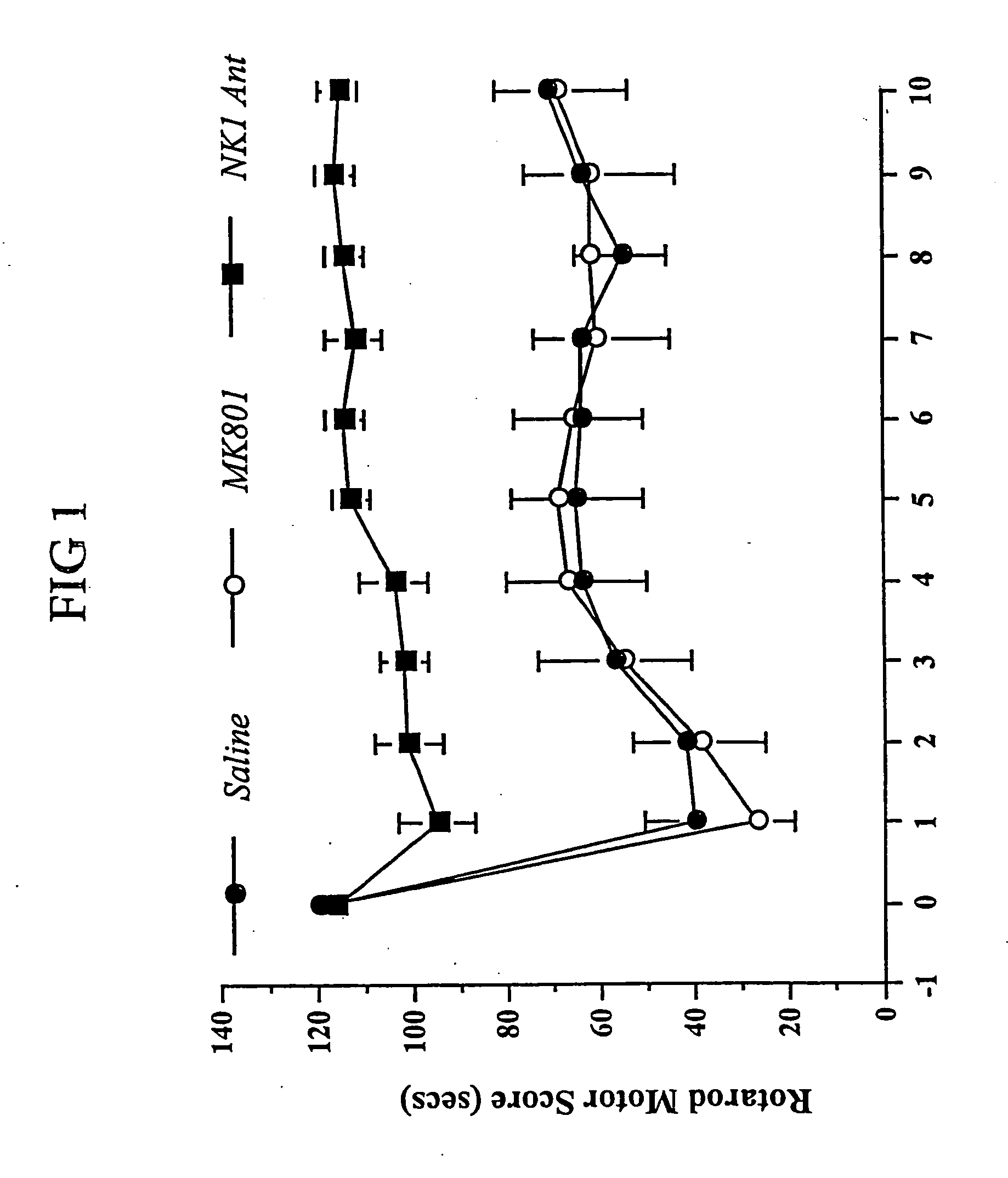
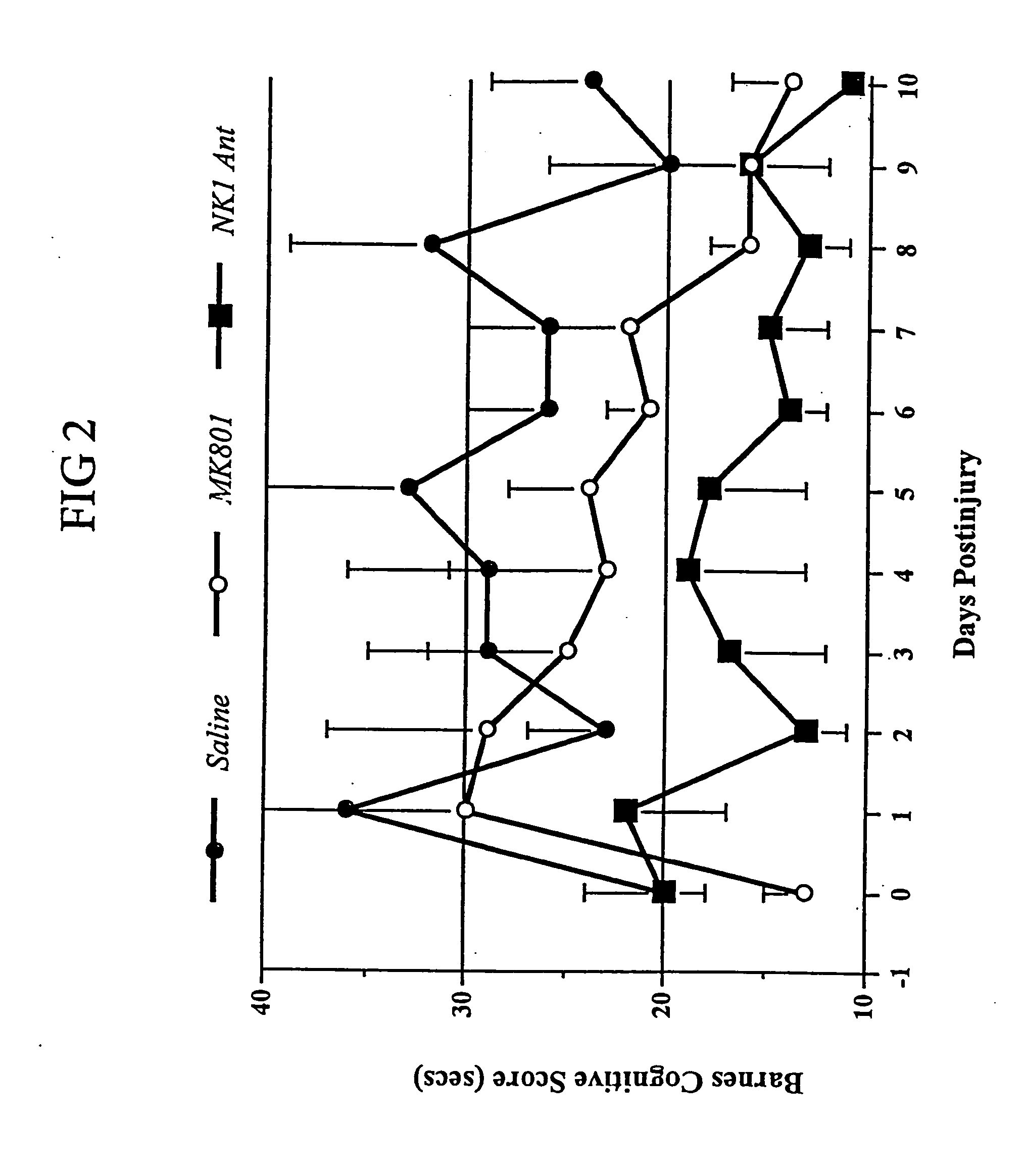
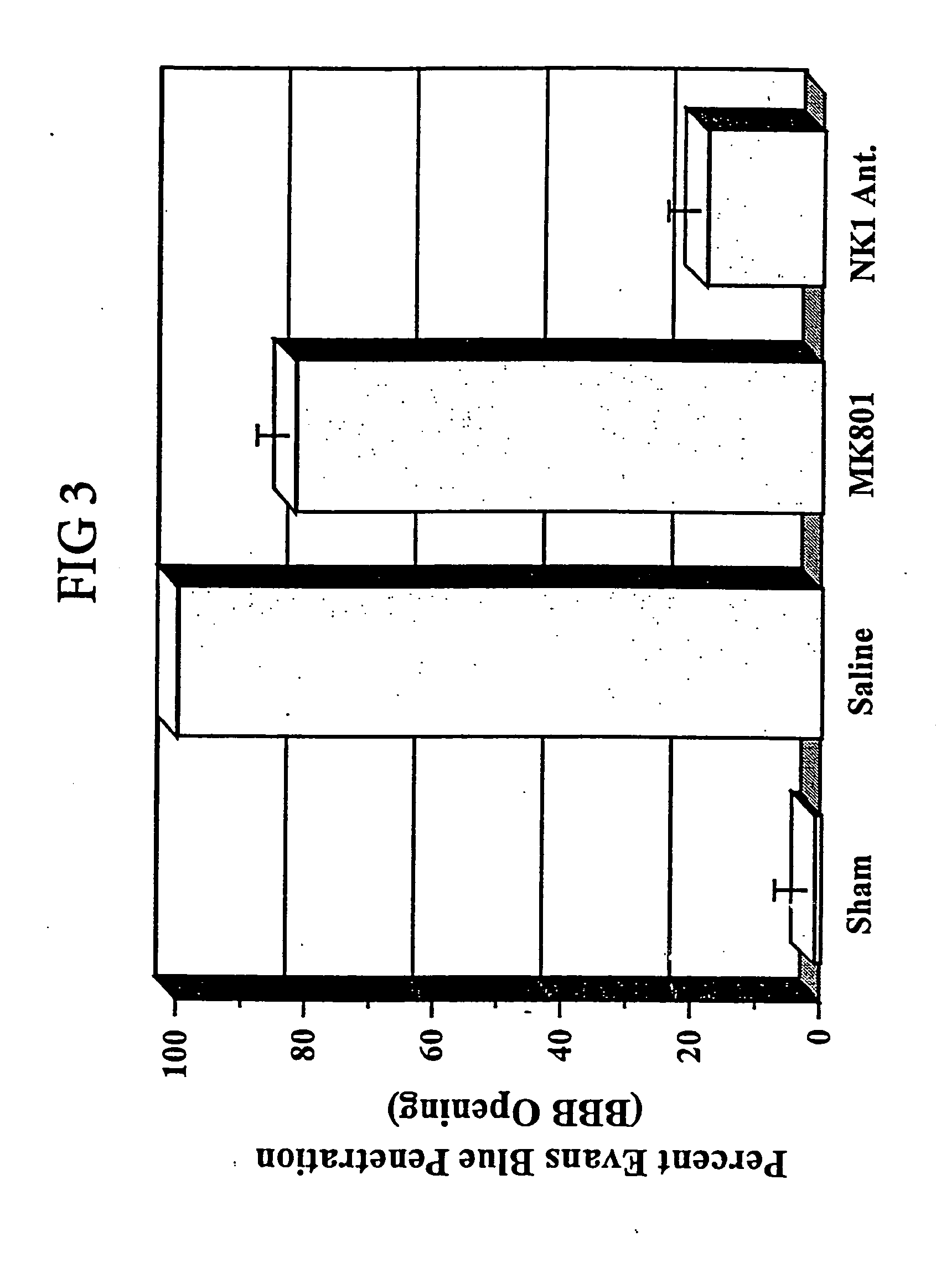
Method of treatment and/or prevention of brain, spinal or nerve injury
InactiveUS20110053954A1Improve actionEnhance neuroprotectiveSenses disorderNervous disorderMagnesium saltSpinal cord
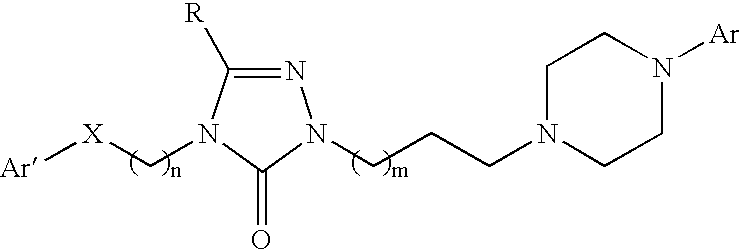
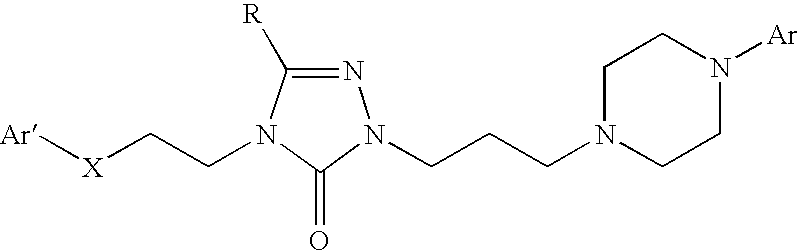
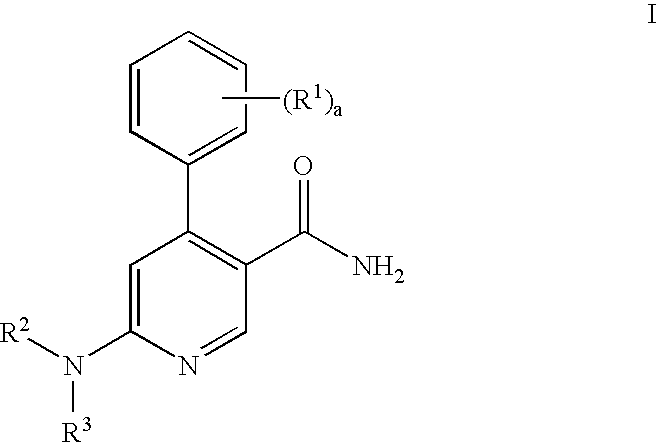
The invention relates to a method of treatment and / or prevention of brain, spinal or nerve injury comprising administration to a person in need of such treatment, of a therapeutically effective amount of an NK-1 receptor antagonist compound of the formulawherein the meanings of R, R1, R2, R2′, R3, and R4 are explained in the specification and the pharmaceutically acceptable acid addition salts and the prodrugs thereof either alone or in combination with a magnesium salt. Exemplified is the use of N-(3,5-bis-trifluoromethyl-benzyl)-N-methyl-6-(4-methyl-piperazin-1-yl)-4-o-tolyl-nicotinamide. The invention also relates to pharmaceutical composition comprising one or more such NK-1 receptor antagonists, optionally in combination with a magnesium salt, and a pharmaceutically acceptable excipient for the treatment and / or prevention of brain, spinal or nerve injury.
Owner:HOFFMANN TORSTEN +4
Method of treatment and/or prevention of brain, spinal or nerve injury
InactiveUS20060247240A1Improve actionEnhance neuroprotectiveBiocideSenses disorderMagnesium saltNK 1 Receptor Antagonists
The invention relates to a method of treatment and / or prevention of brain, spinal or nerve injury comprising administration to a person in need of such treatment, of a therapeutically effective amount of an NK-1 receptor antagonist compound of the formula wherein the meanings of R, R1, R2, R2, R3, and R4 are explained in the specification and the pharmaceutically acceptable acid addition salts and the prodrugs thereof either alone or in combination with a magnesium salt. Exemplified is the use of N-(3,5-bis-trifluoromethyl-benzyl)-N-methyl-6-(4-methyl-piperazin-1-yl)-4-o-tolyl-nicotinamide. The invention also relates to pharmaceutical composition comprising one or more such NK-1 receptor antagonists, optionally in combination with a magnesium salt, and a pharmaceutically acceptable excipient for the treatment and / or prevention of brain, spinal or nerve injury.
Owner:HOFFMANN TORSTEN +4
New itch treatment using a combination of neurokinin-1, gastrin releasing peptide, and glutamate receptor antagonists
InactiveUS20150320827A1Avoid spreadingReduce excitementBiocidePeptide/protein ingredientsGastrin-releasing peptideNK1 receptor antagonist
Methods, and compositions are provided for inhibition of histamine and non-histamine dependent itch signal transmission or scratch behavior. In one aspect, the present invention further comprises administering to the subject an inhibitor of histamine-dependent itch signal transmission. In some cases, the inhibitor of histamine independent itch signal transmission comprises an NK-1 receptor antagonist or the inhibitor of histamine independent itch signal transmission comprises a GRP receptor antagonist. In some cases, the method comprises administering two inhibitors of histamine independent itch signal transmission. For example, the inhibitors of histamine independent itch signal transmission can comprise an NK-1 receptor antagonist and a GRP receptor antagonist. In another embodiment, the invention provides a method of treating itch comprising administering to a subject suffering from itch an NK-1 receptor antagonist, a GRP receptor antagonist.
Owner:RGT UNIV OF CALIFORNIA
Use of NK-1 receptor antagonist serlopitant in pruritus
InactiveCN105473138AOrganic active ingredientsNervous disorderAntipruritic agentsNK 1 Receptor Antagonists
The invention relates to methods for treating pruritus with NK-1 receptor antagonists such as serlopitant. The invention further relates to pharmaceutical compositions comprising NK-1 receptor antagonists such as serlopitant. In addition, the invention encompasses treatment of a pruritus-associated condition with serlopitant and an additional antipruritic agent, and the use of serlopitant as a sleep aid, optionally in combination with an additional sleep-aiding agent.
Owner:MENLO THERAPEUTICS INC
Tachykinin receptor antagonists
The present invention relates to selective NK-1 receptor antagonists of Formula (I); or a pharmaceutically acceptable salt thereof, for the treatment of disorders associated with an excess of tachykinins.
Owner:ELI LILLY & CO
Treatment of corneal neovascularization
There is provided inter alia a compound which is an NK-1 receptor antagonist for use in the treatment or prevention of CNV. There is also provided a compound which is an NK-1 antagonist for use in the treatment of chemical burns of the eye particularly alkali burns of the eye. There is also provided a pharmaceutical composition for topical administration to the eye comprising an NK-1 antagonist and an antibiotic agent.
Owner:IRBM SCI PARK
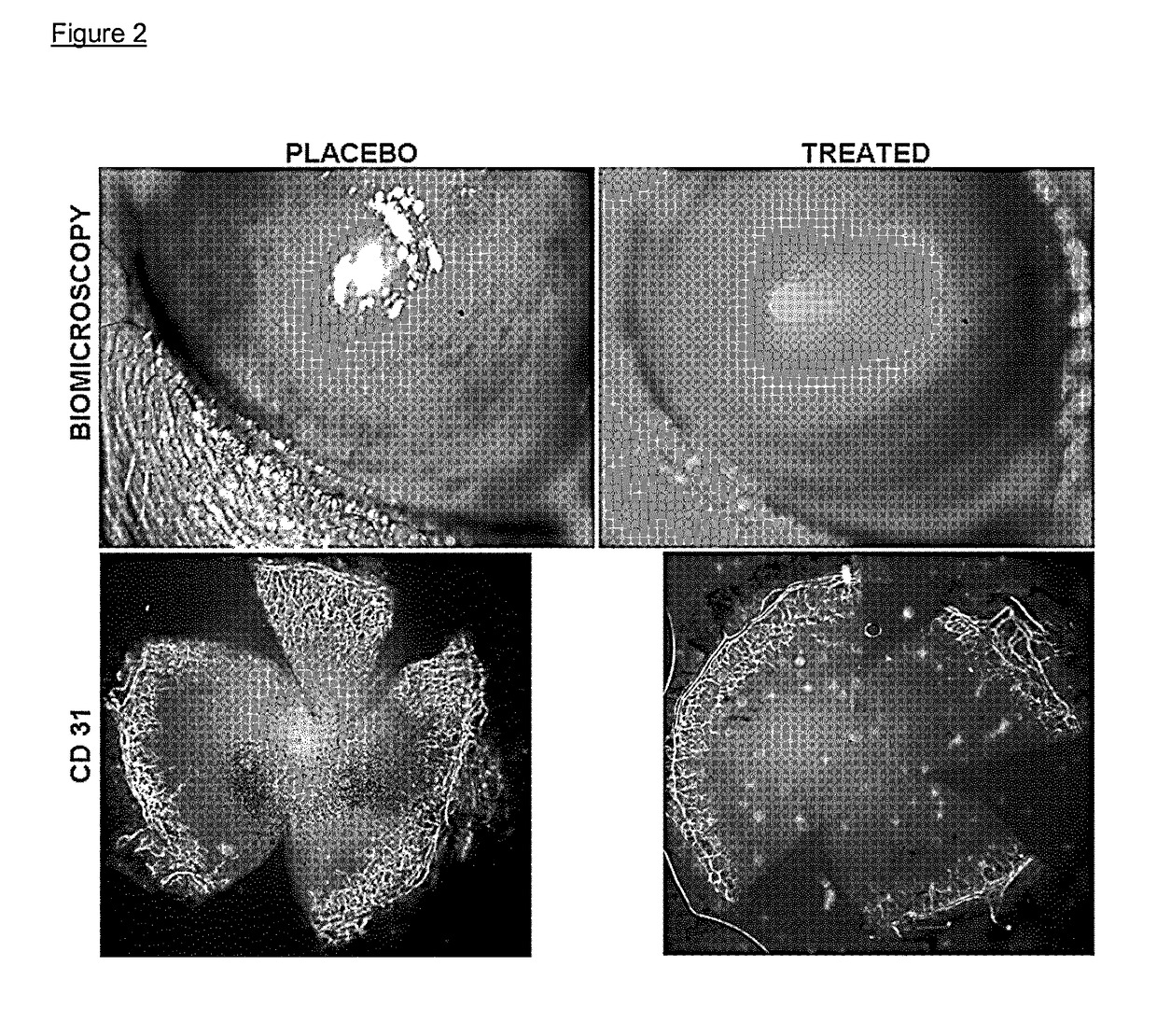
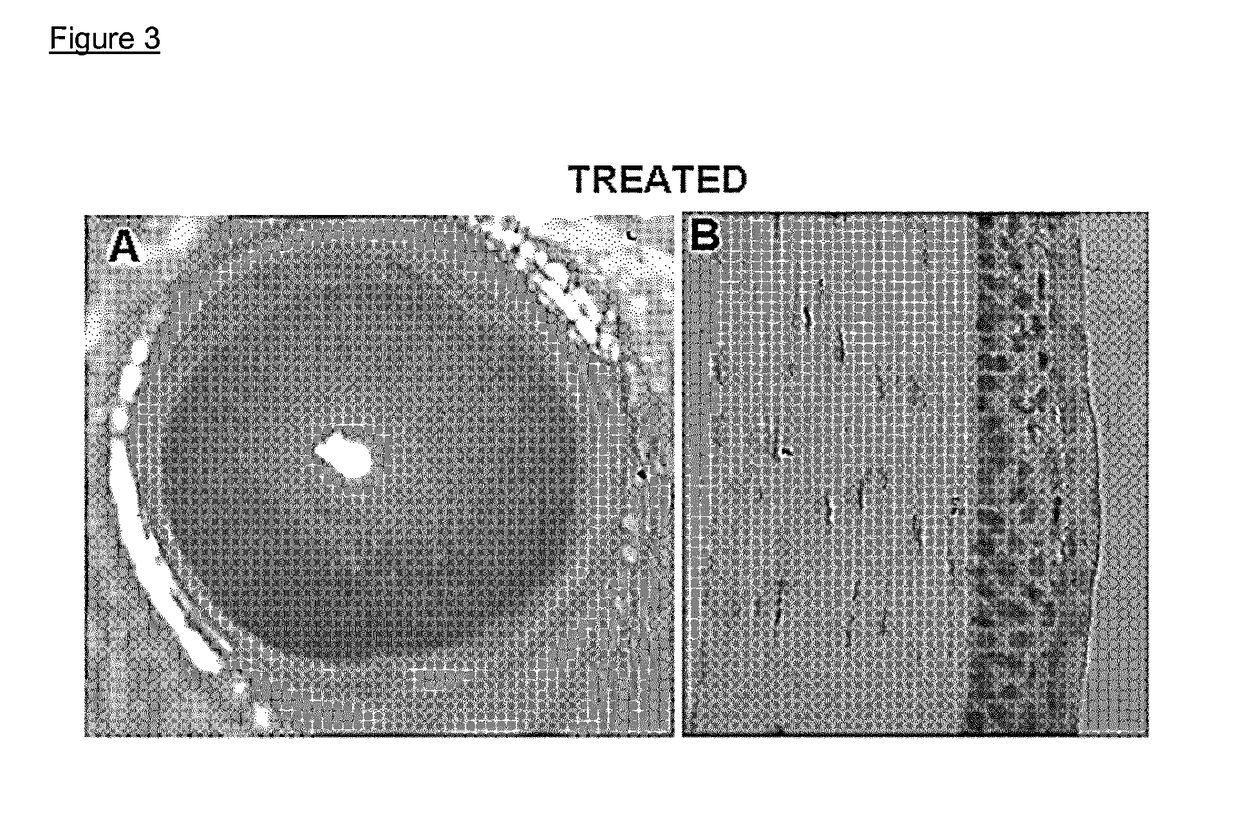
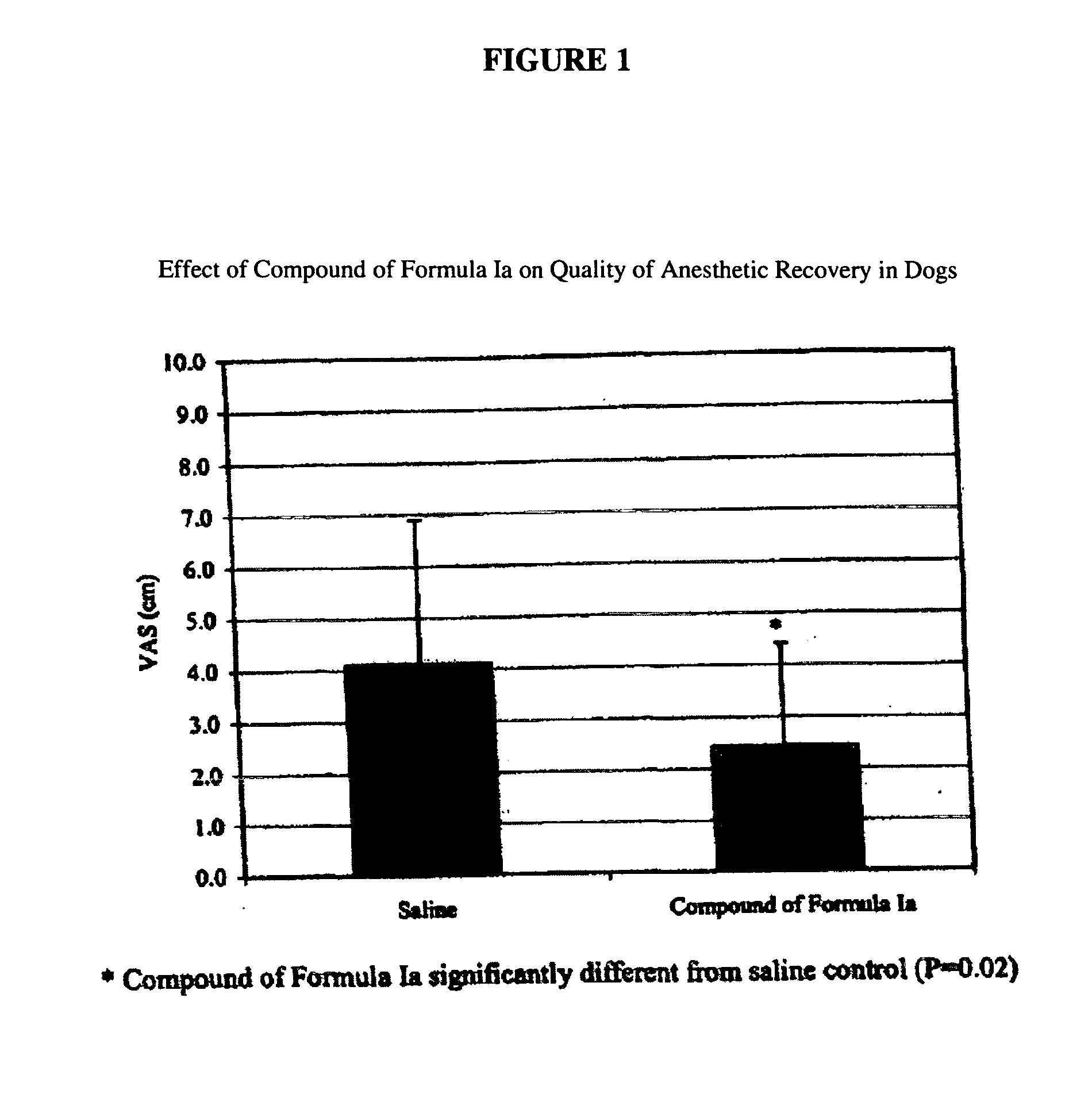
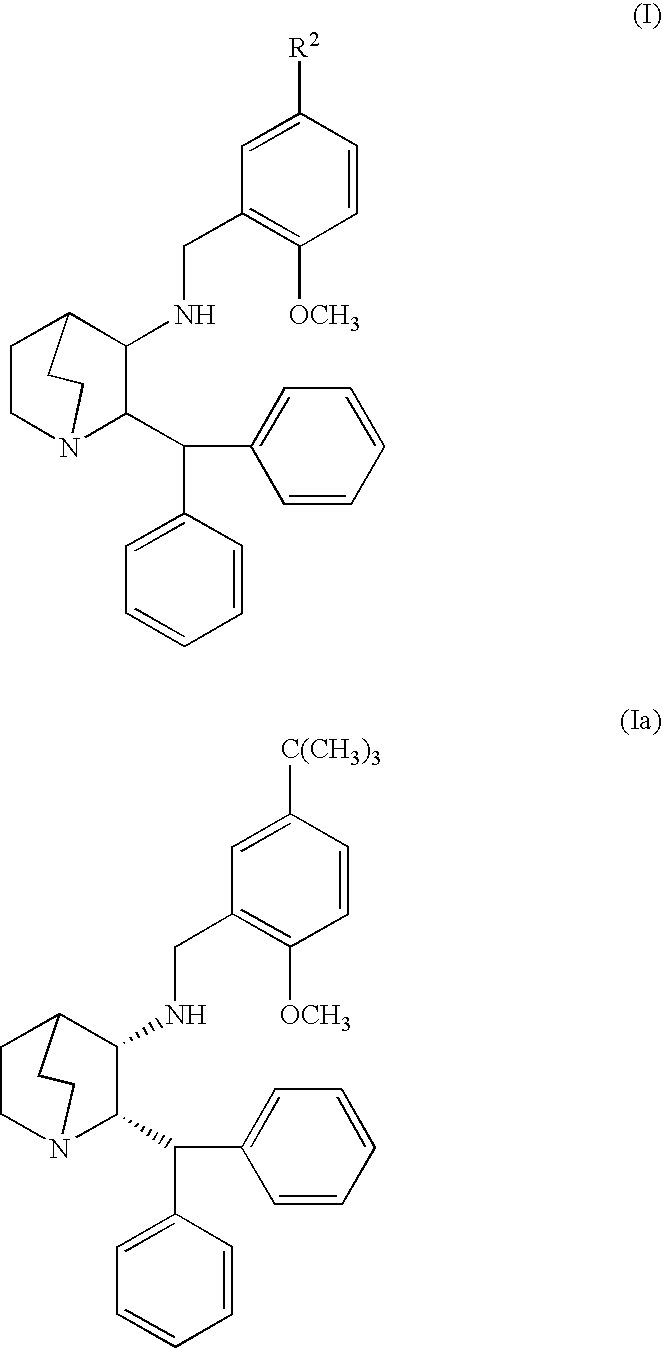
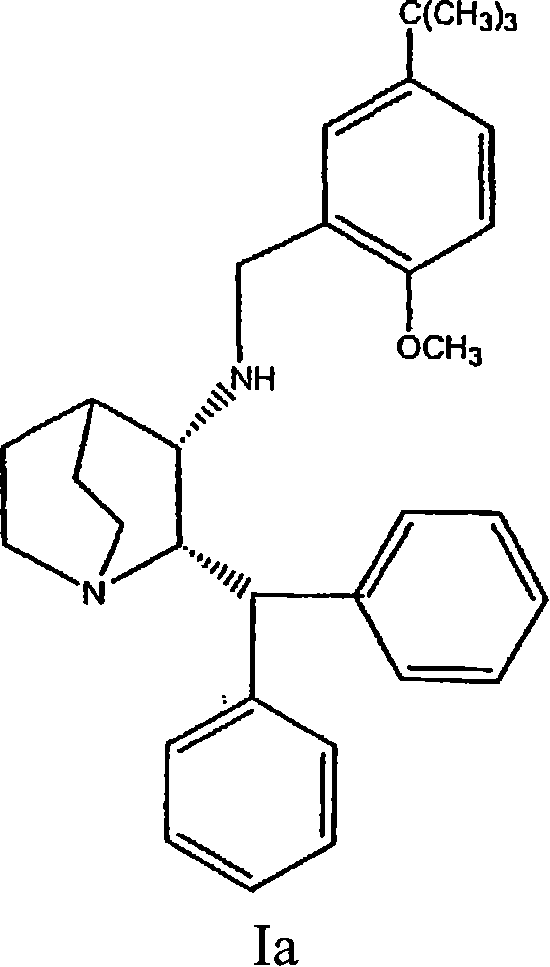
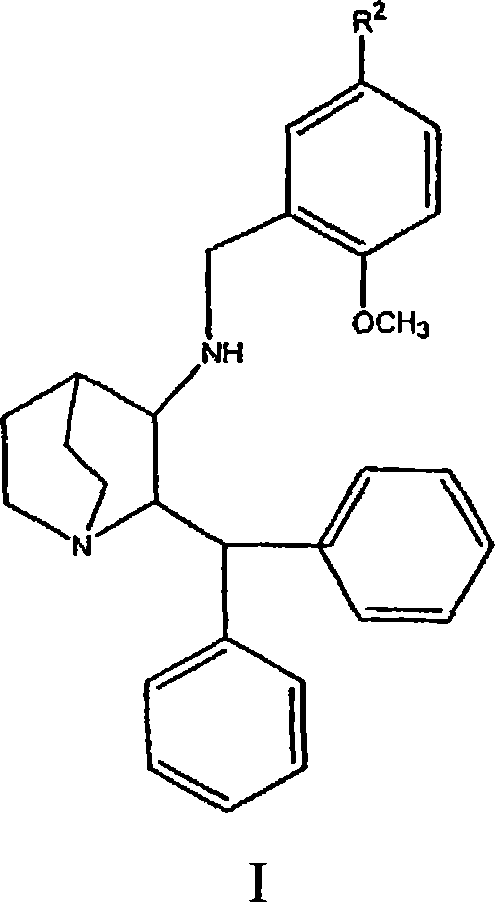
Nk-1 receptor antagonists anesthesia recovery
InactiveUS20070155782A1Promote recoveryQuality improvementBiocideNervous disorderAnesthesiaNK 1 Receptor Antagonists
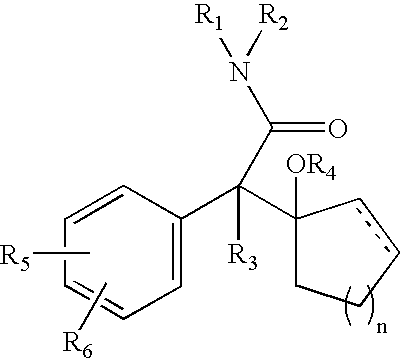
The present invention is directed to the administration of a compound of the Formula (I) and (Ia), wherein R2 is selected from the group consisting of methyl, ethyl, isopropyl, sec-butyl and tert-butyl, to an animal to improve anesthesia recovery
Owner:HICKMAN MARY ANNE +1
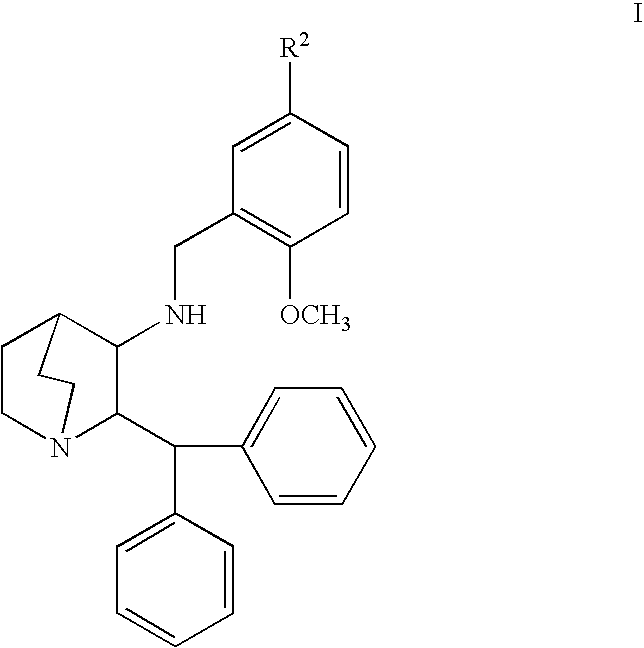
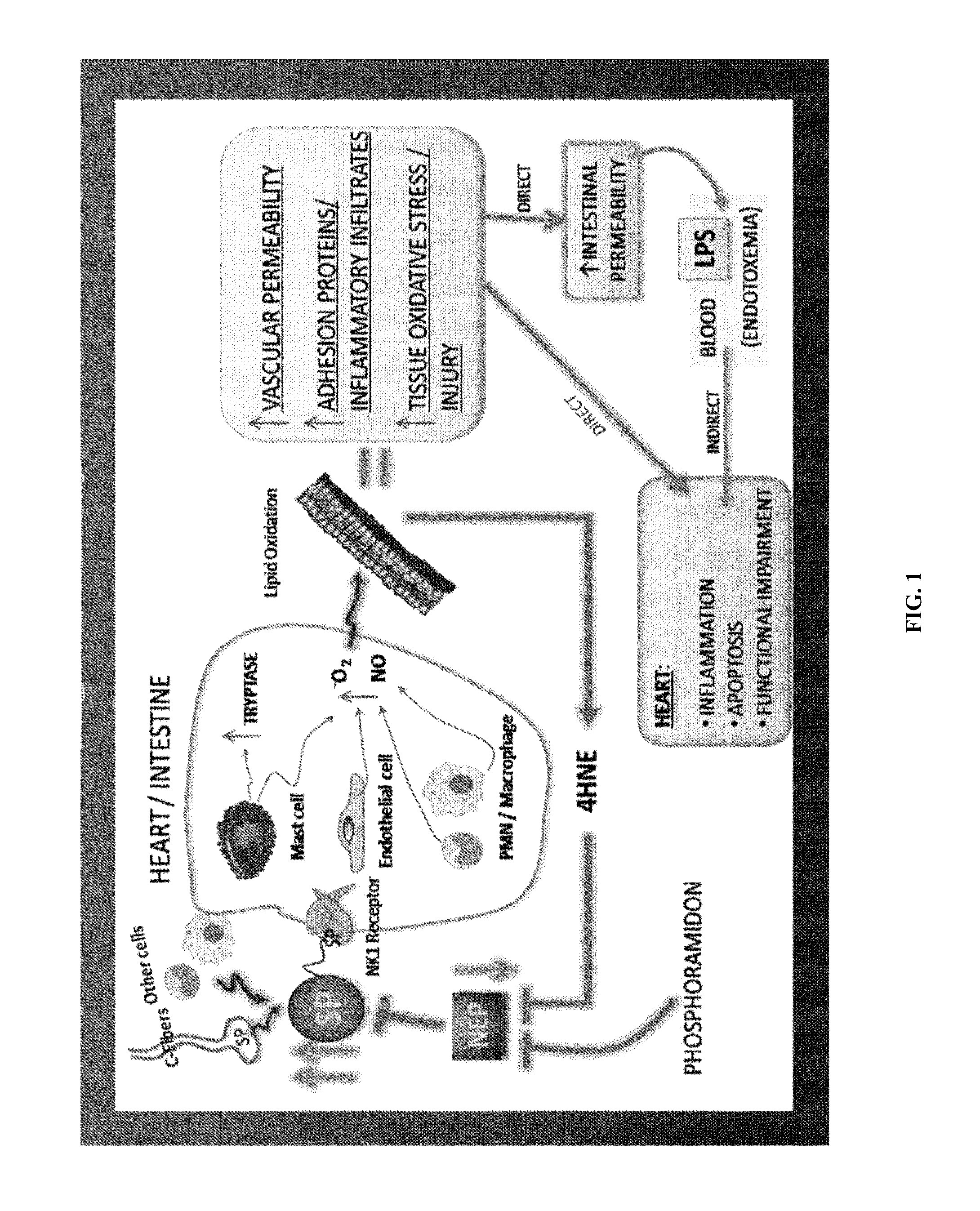
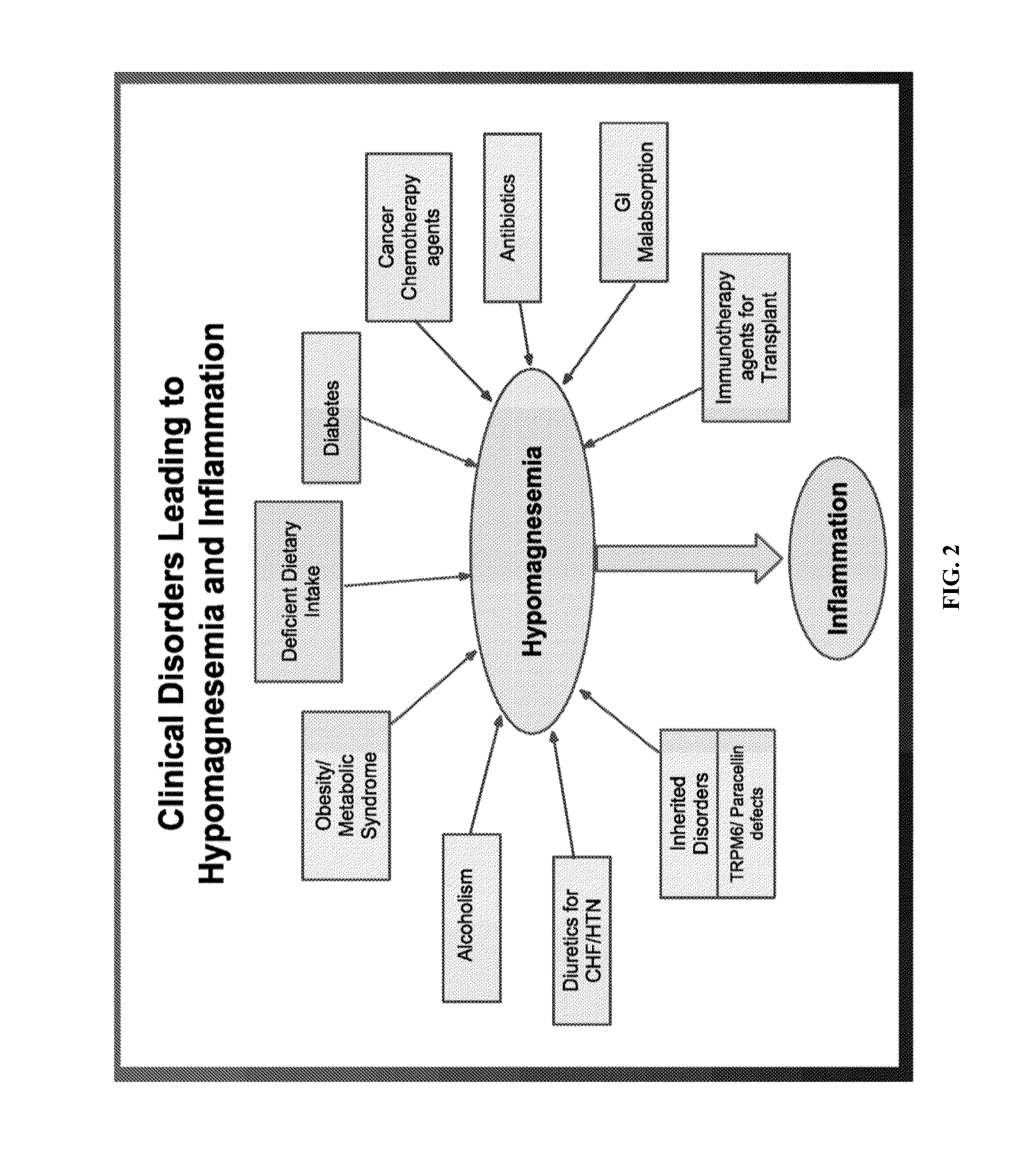
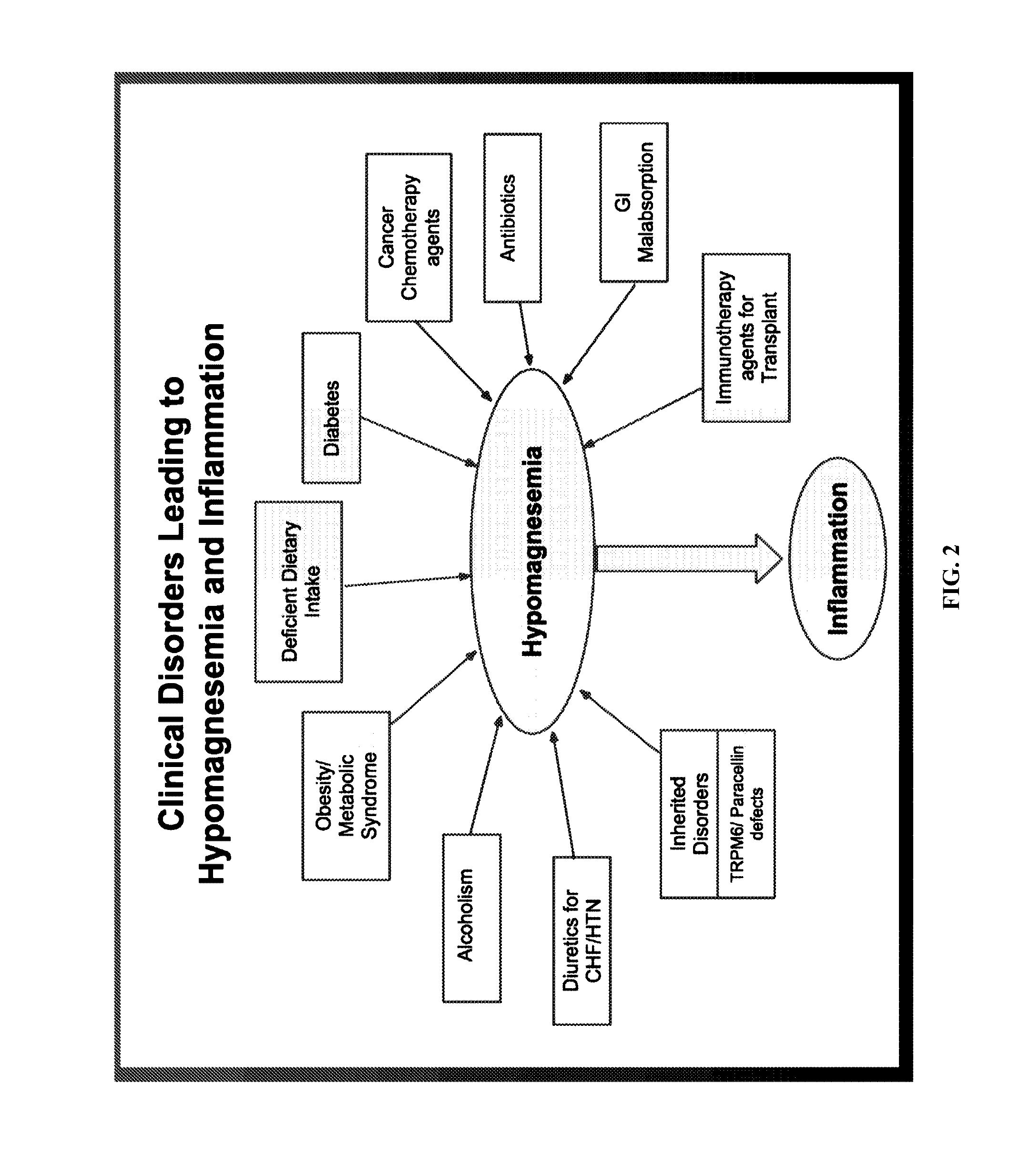
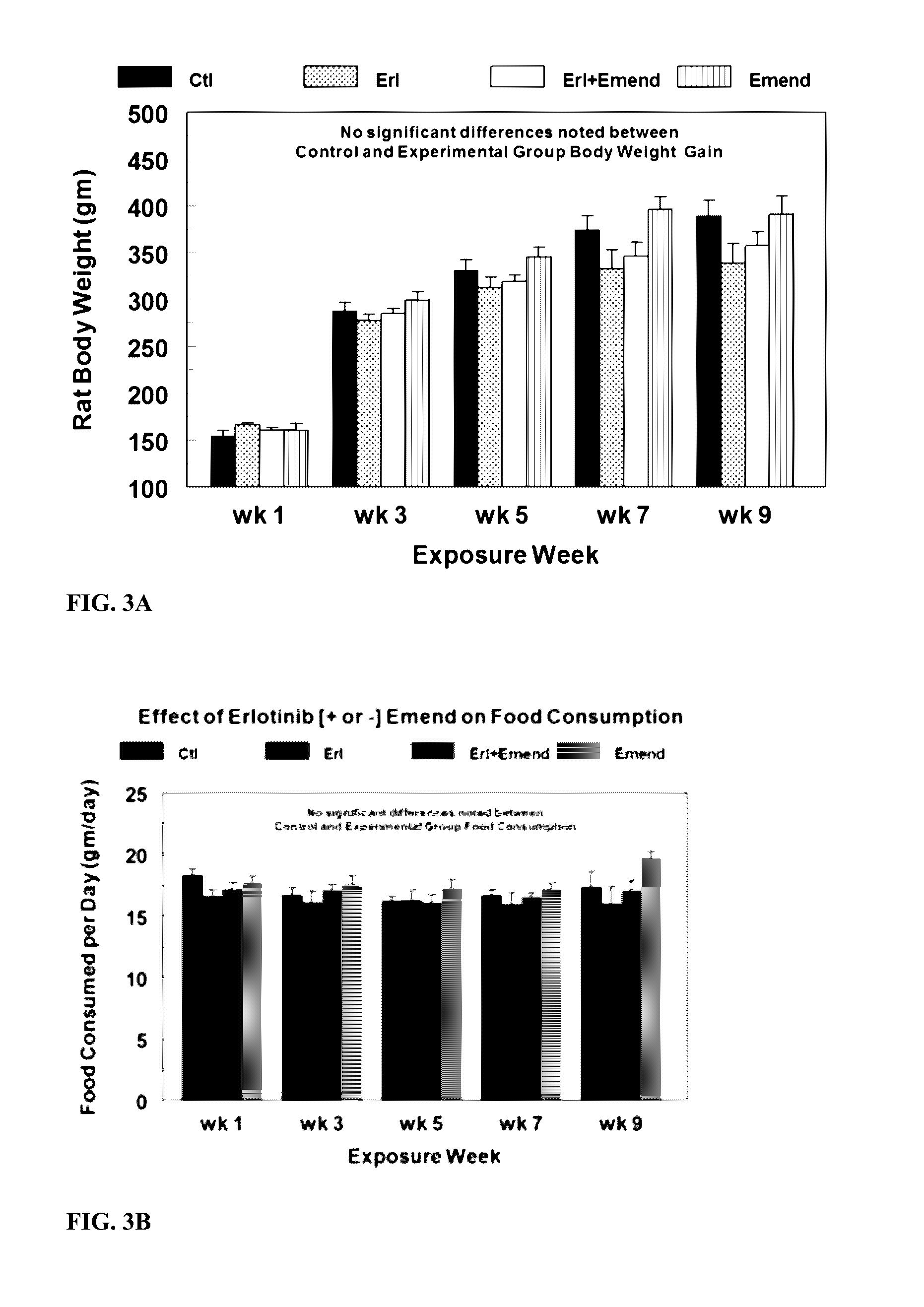
Use of nk-1 receptor antagonists for treating hypomagnesemia, neurogenic inflammation, and cardiac dysfunction associated with egfr-blocking drugs
ActiveUS20150352120A1Effectively diminish, alleviate, or otherwise preventNegative side effectBiocideAnimal repellantsCardiac dysfunctionSide effect
The present disclosure provides methods for alleviating or preventing the negative physiological side effects associated with the administration of EGFR blocking therapeutics. The disclosure provides, inter alia, methods for treating or preventing: hypomagnesemia, cardiac dysfunction, and skin lesions, which are induced by EGFR blocking drugs, by administering an NK-1 receptor antagonist.
Owner:GEORGE WASHINGTON UNIVERSITY
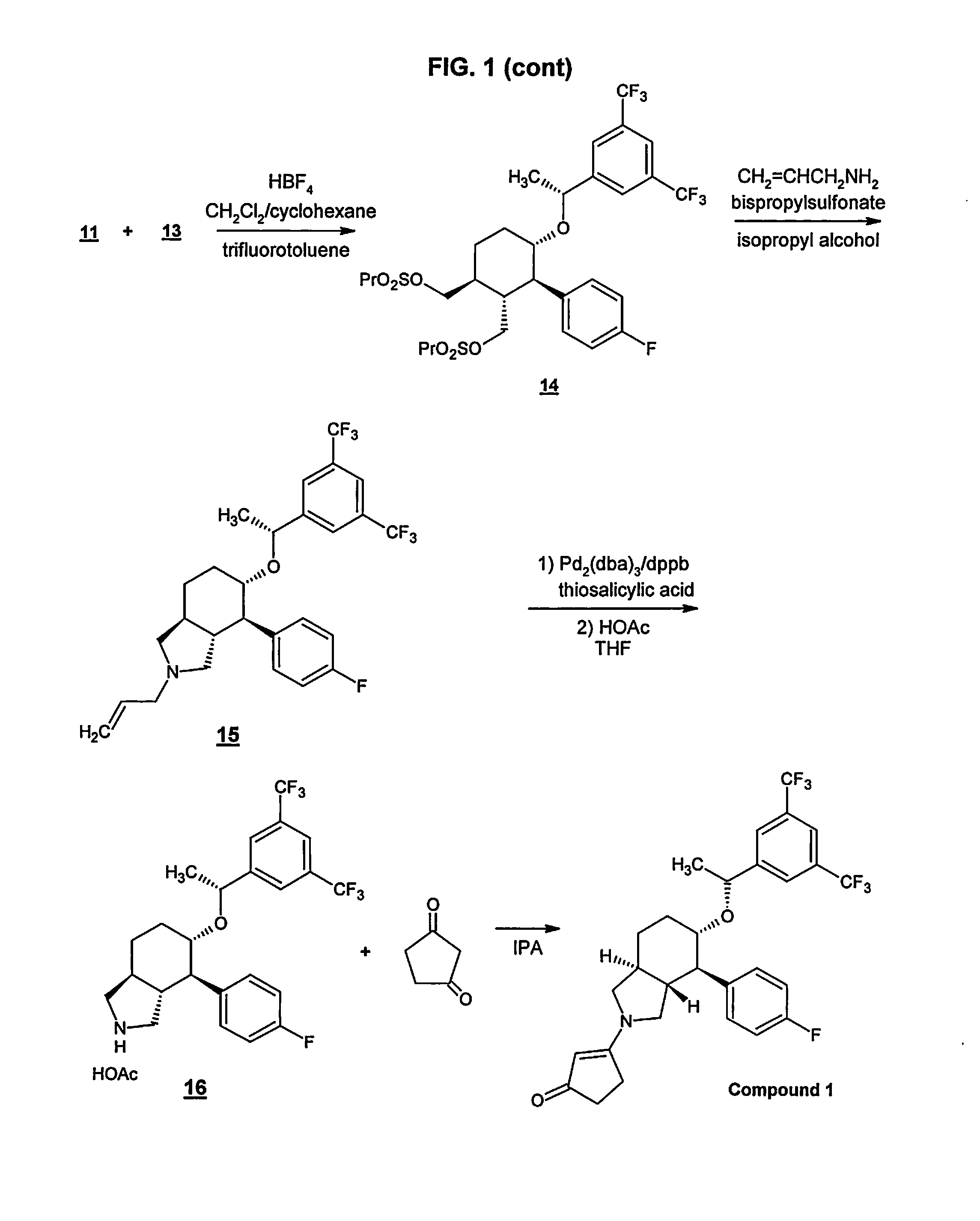
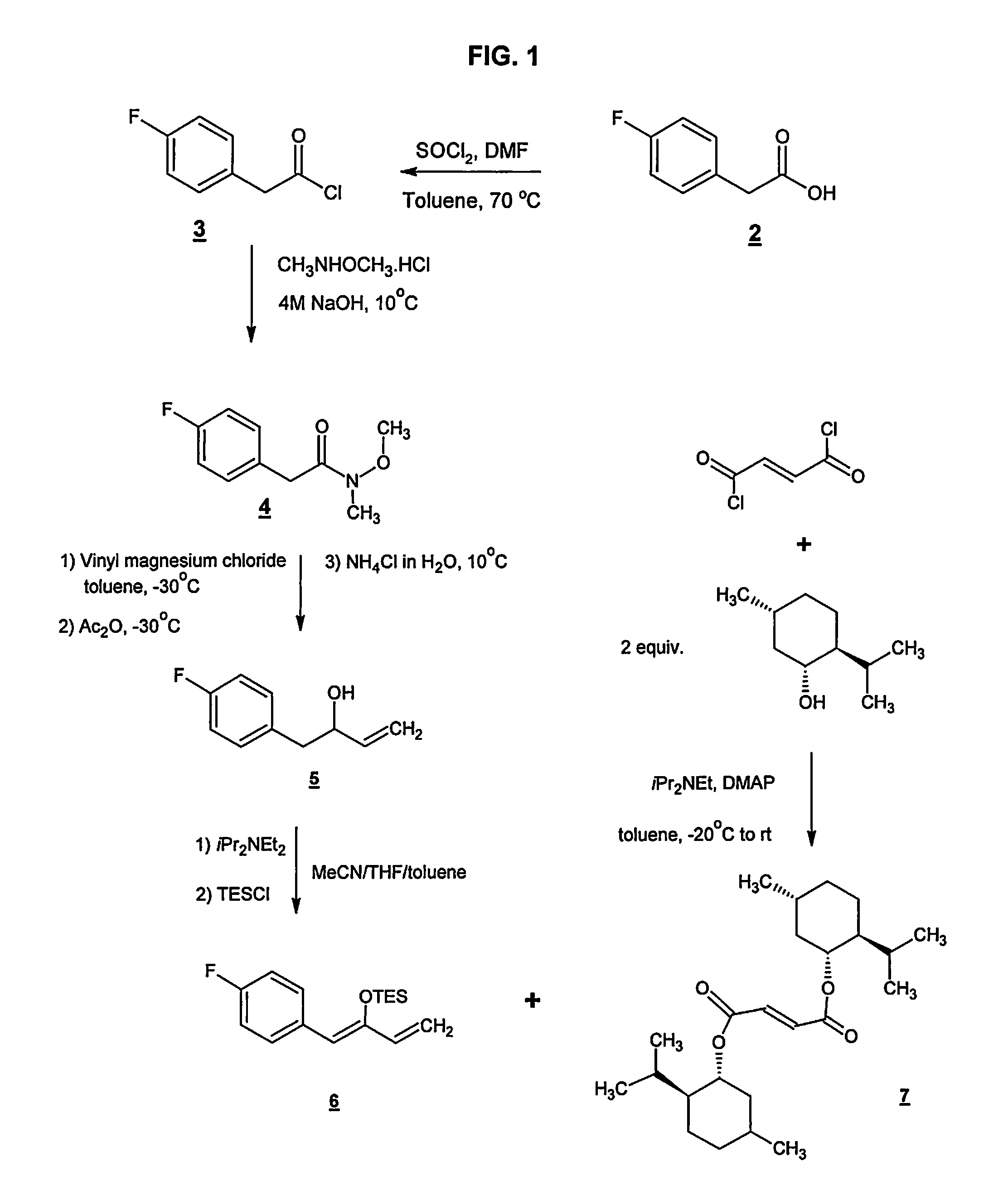
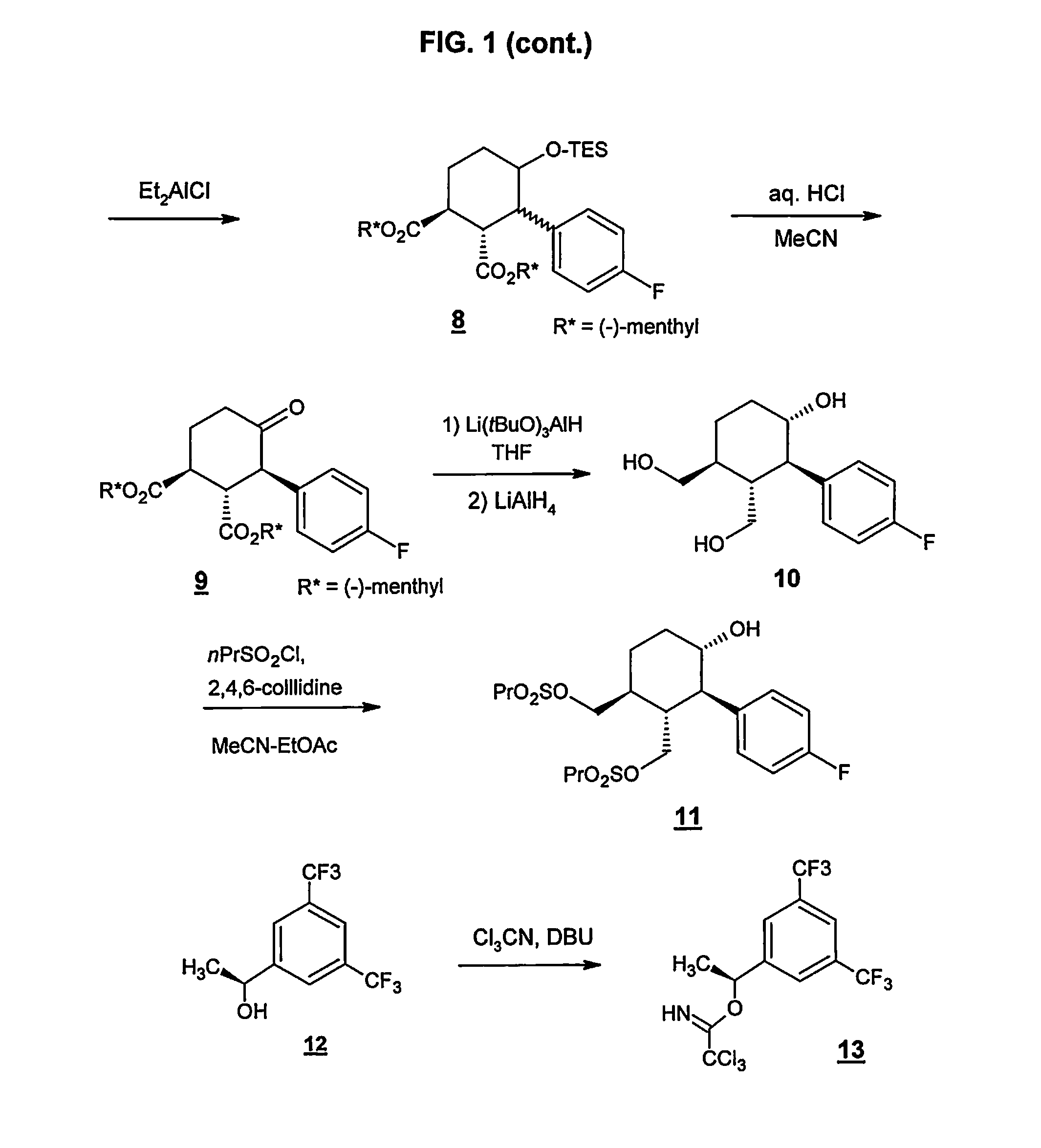
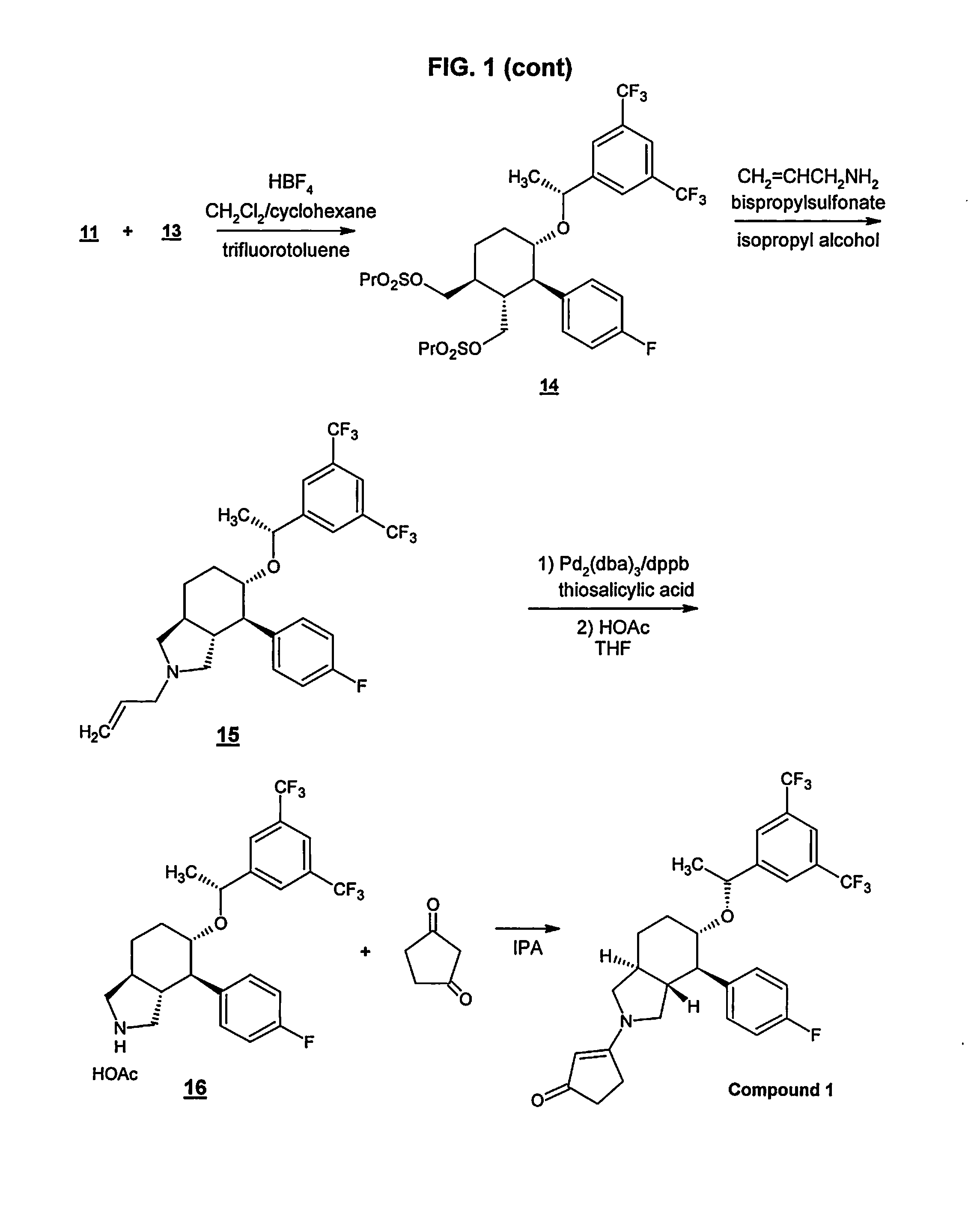
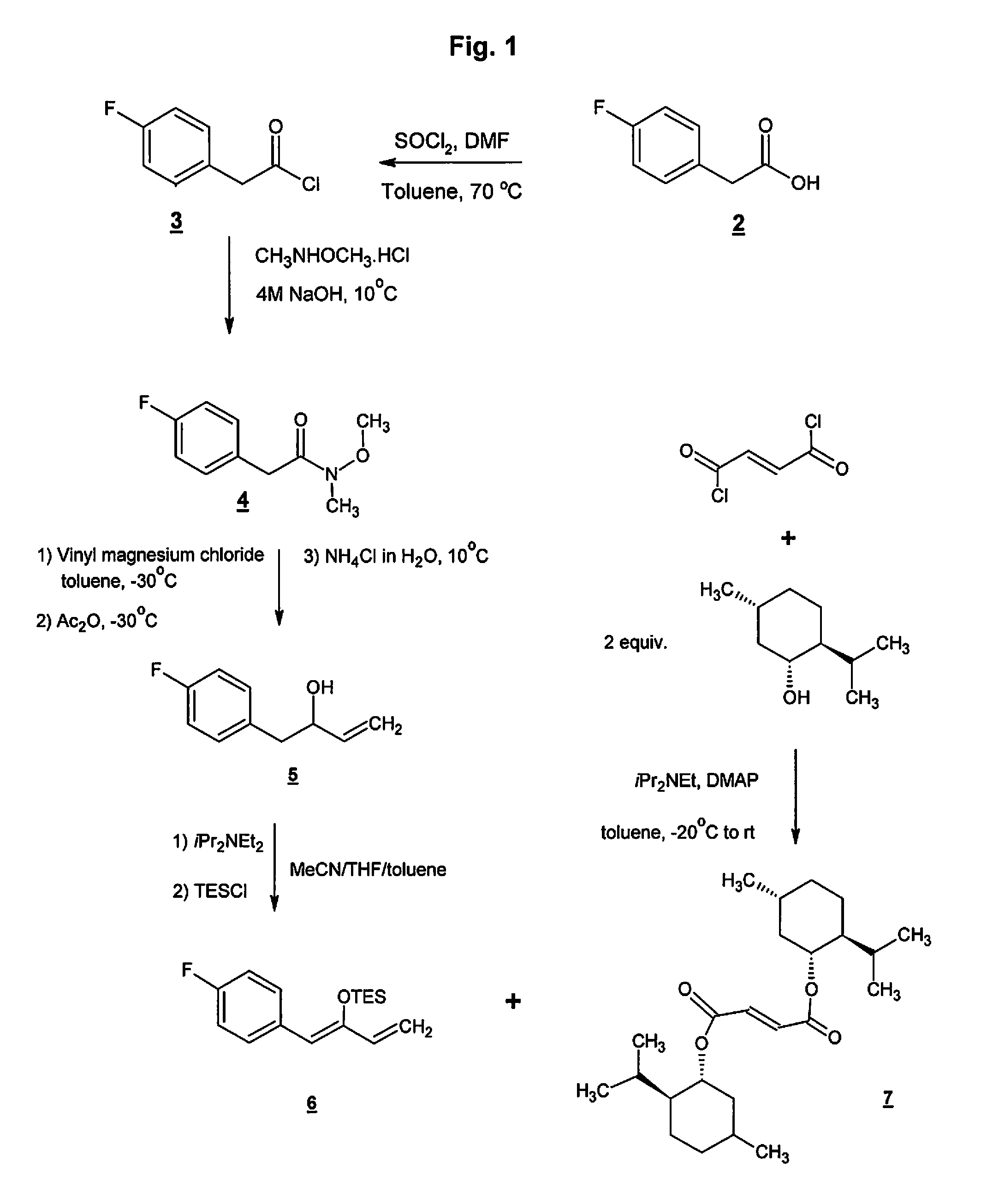
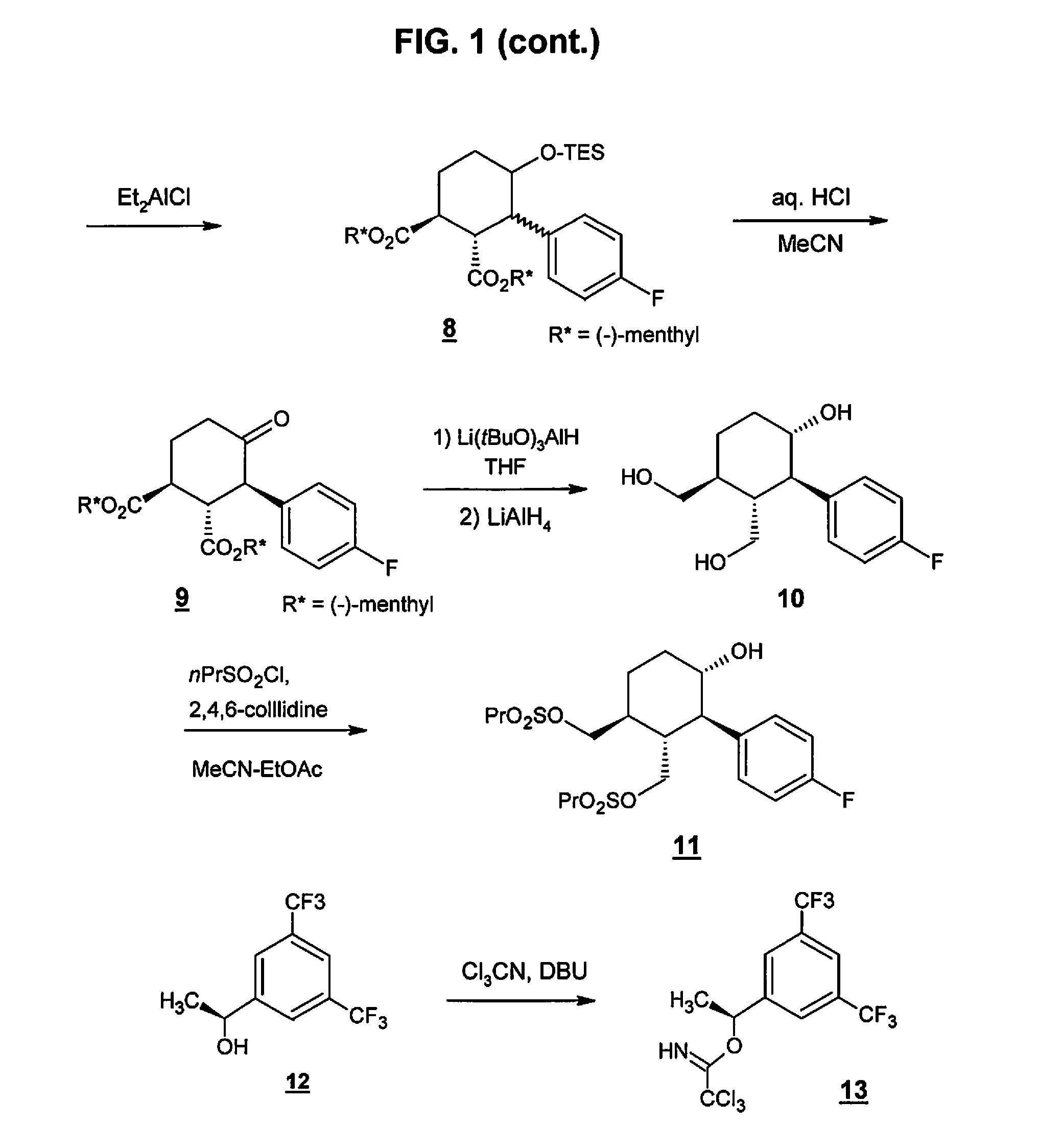
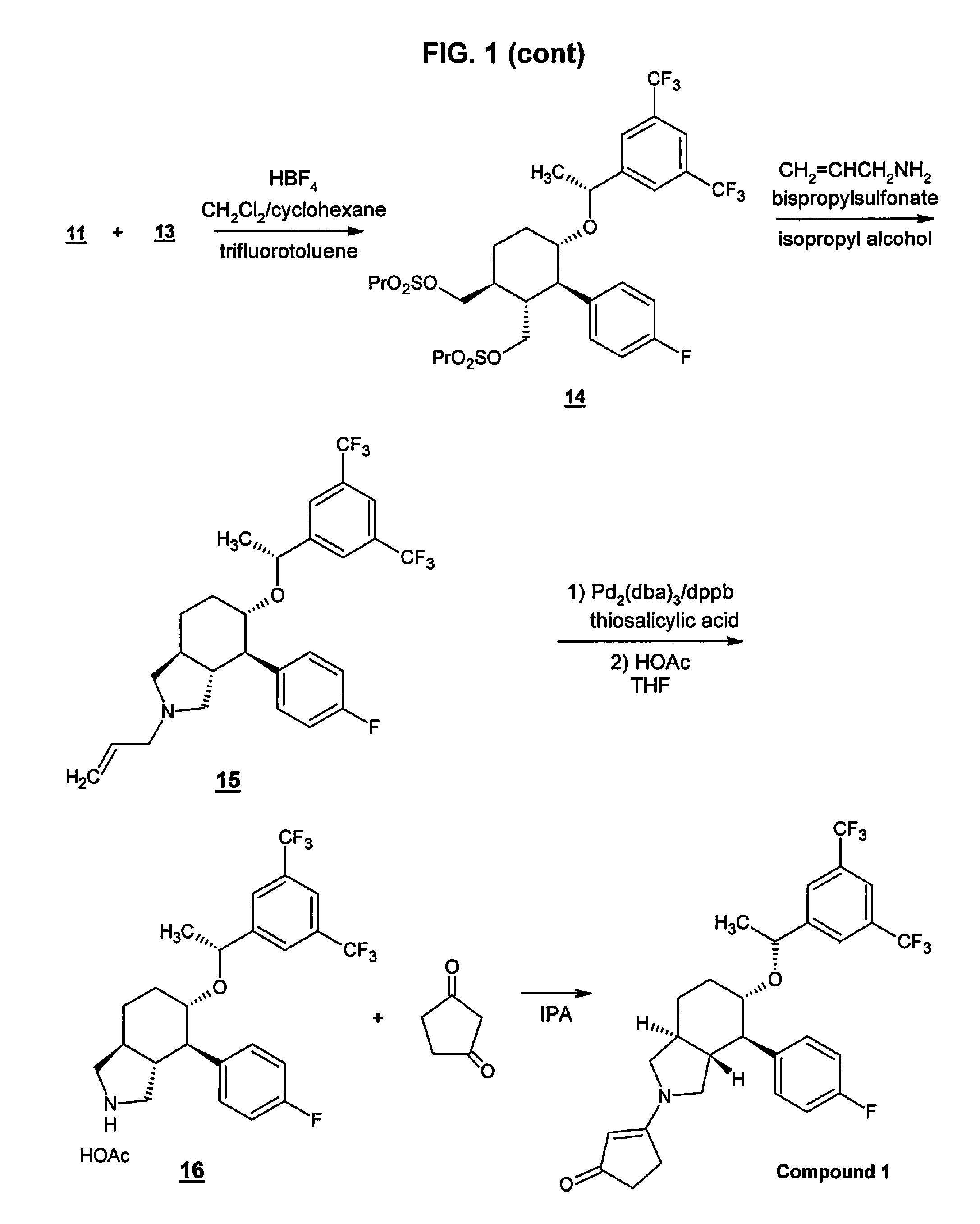
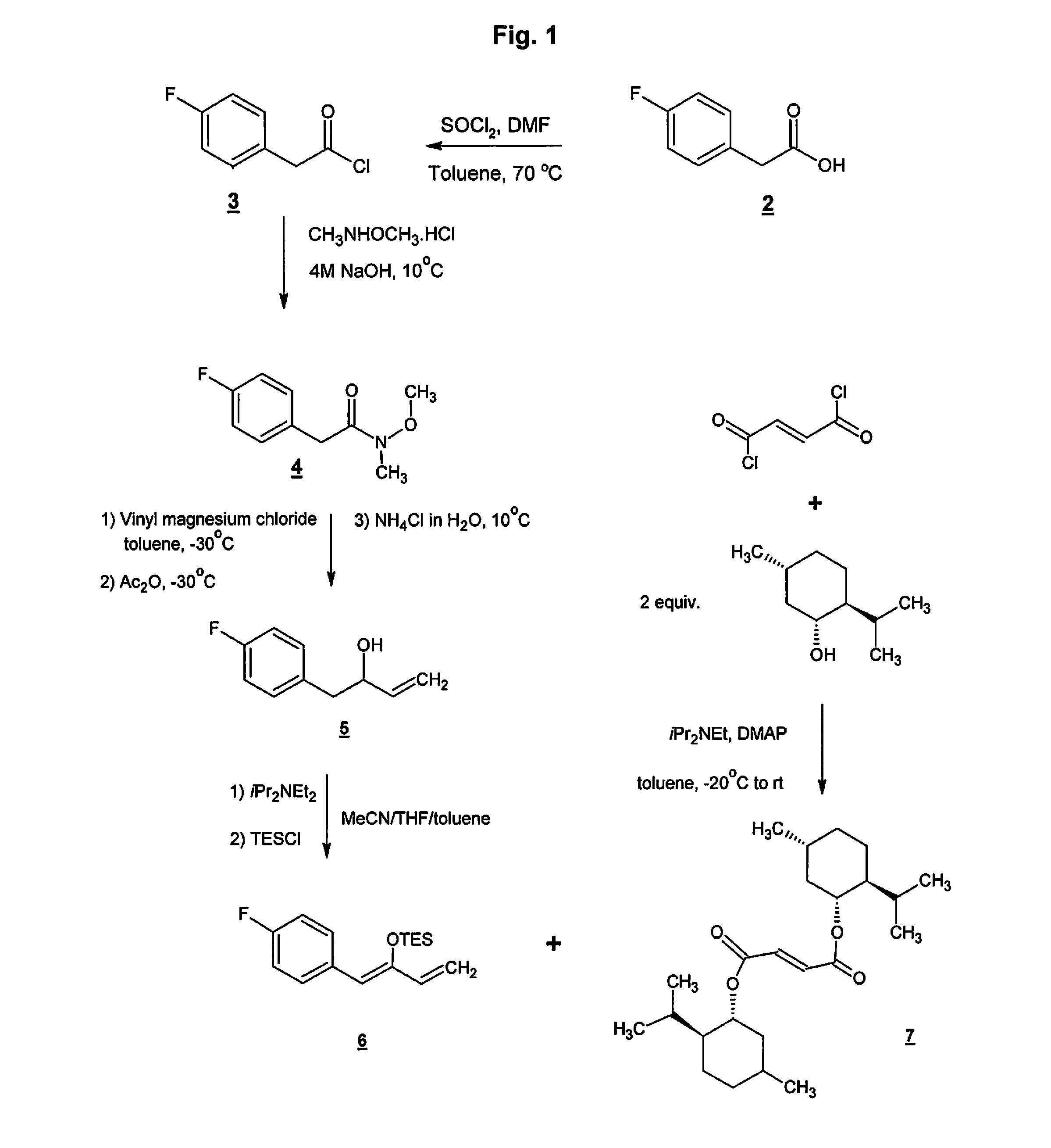
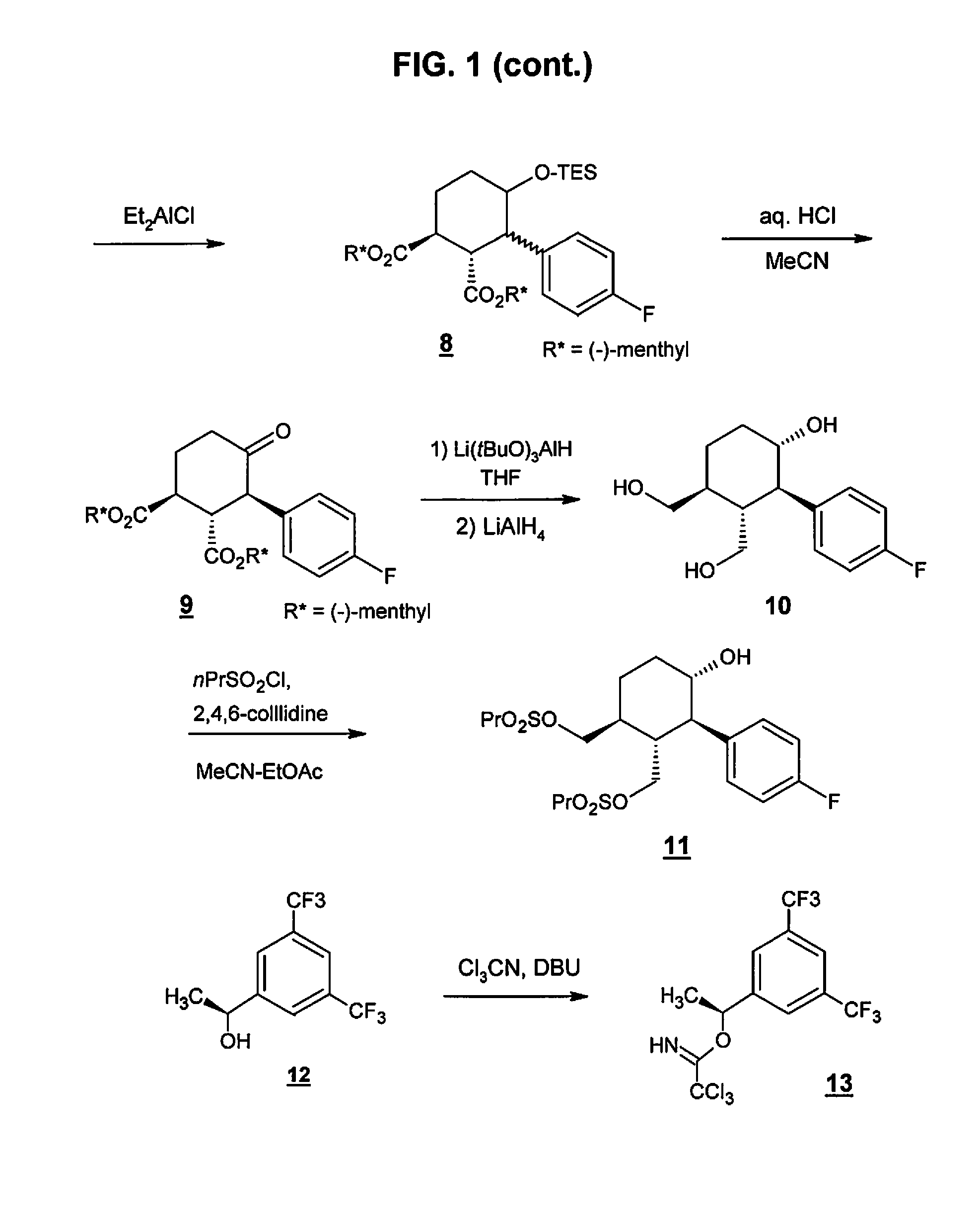
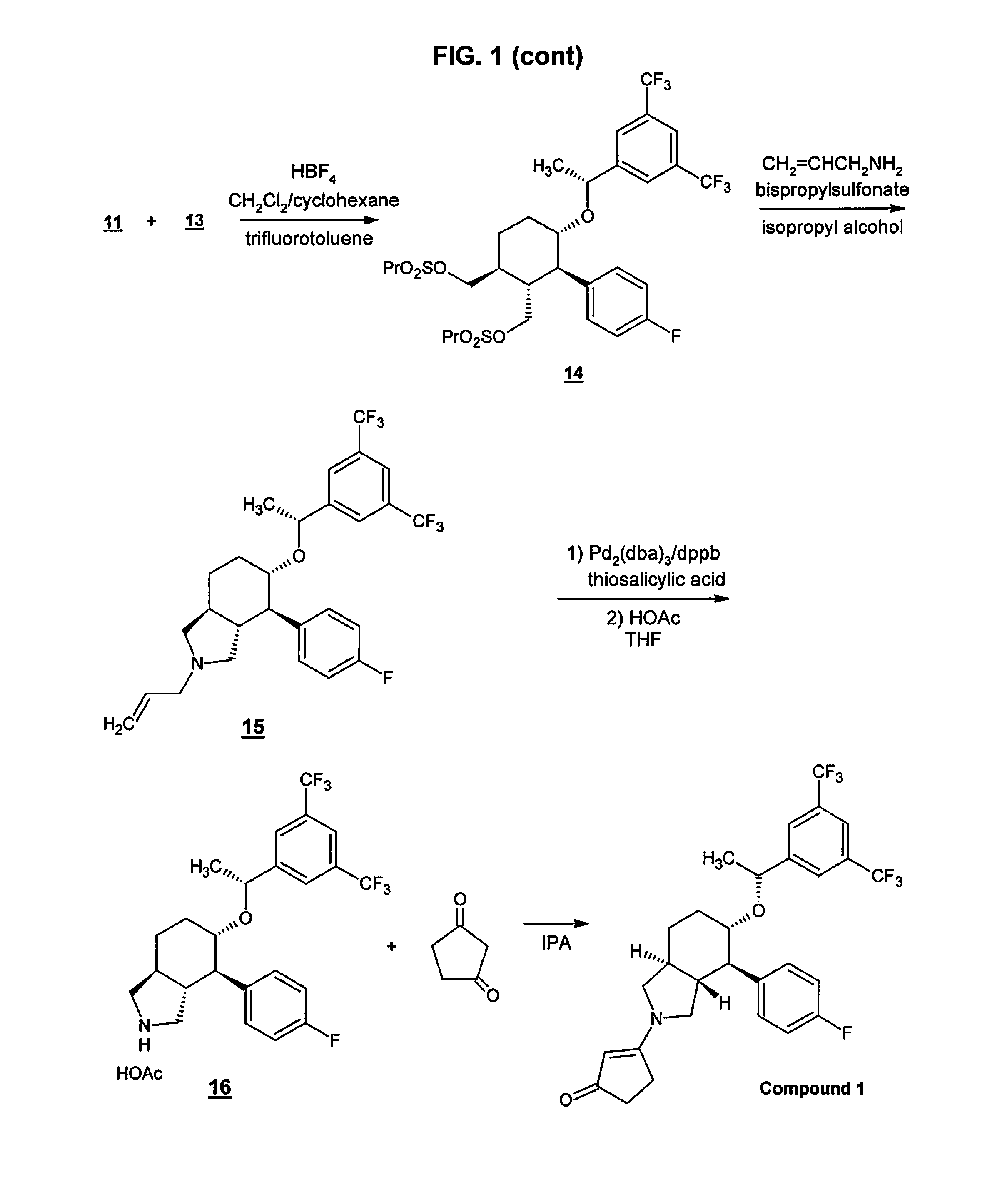
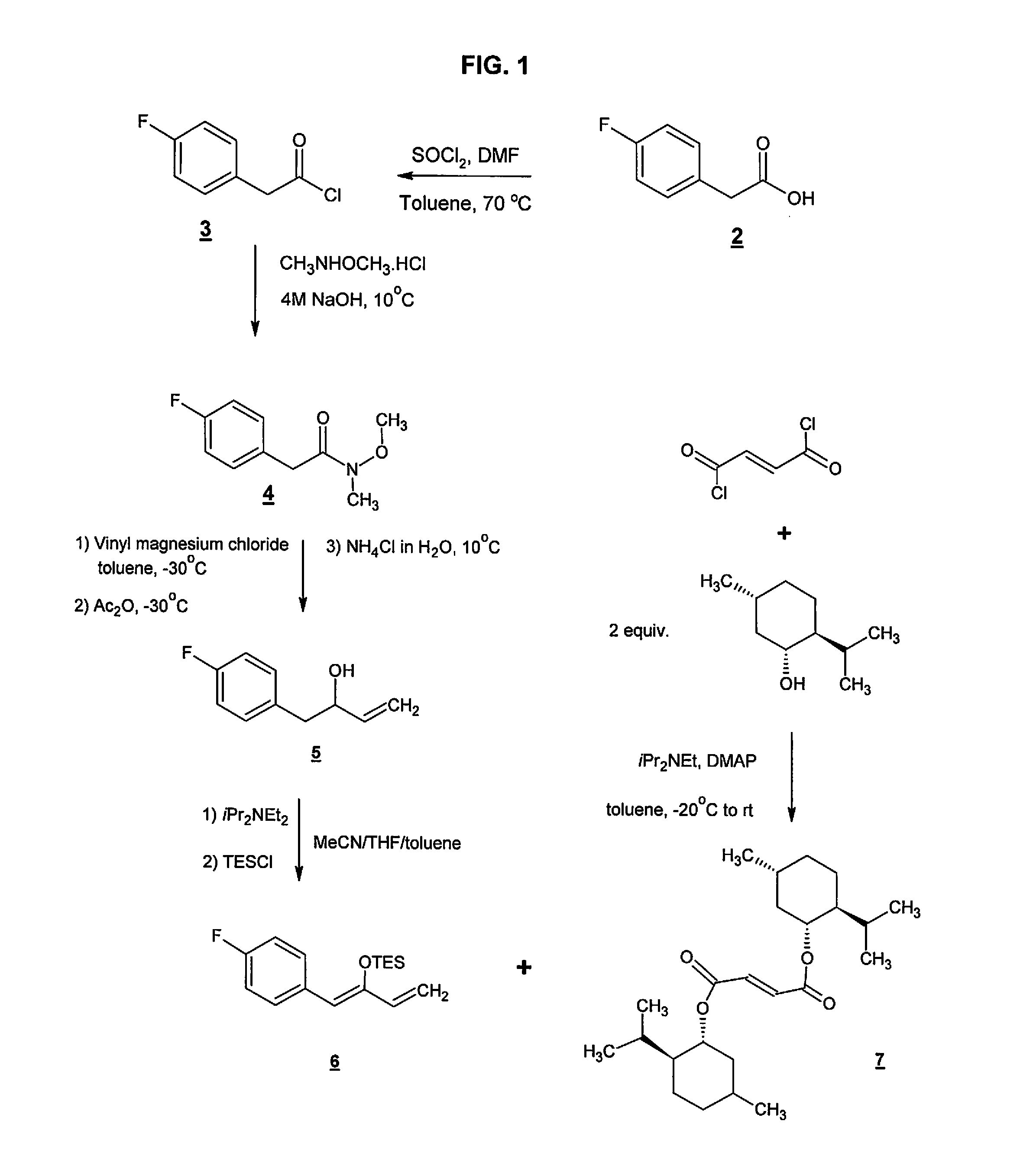
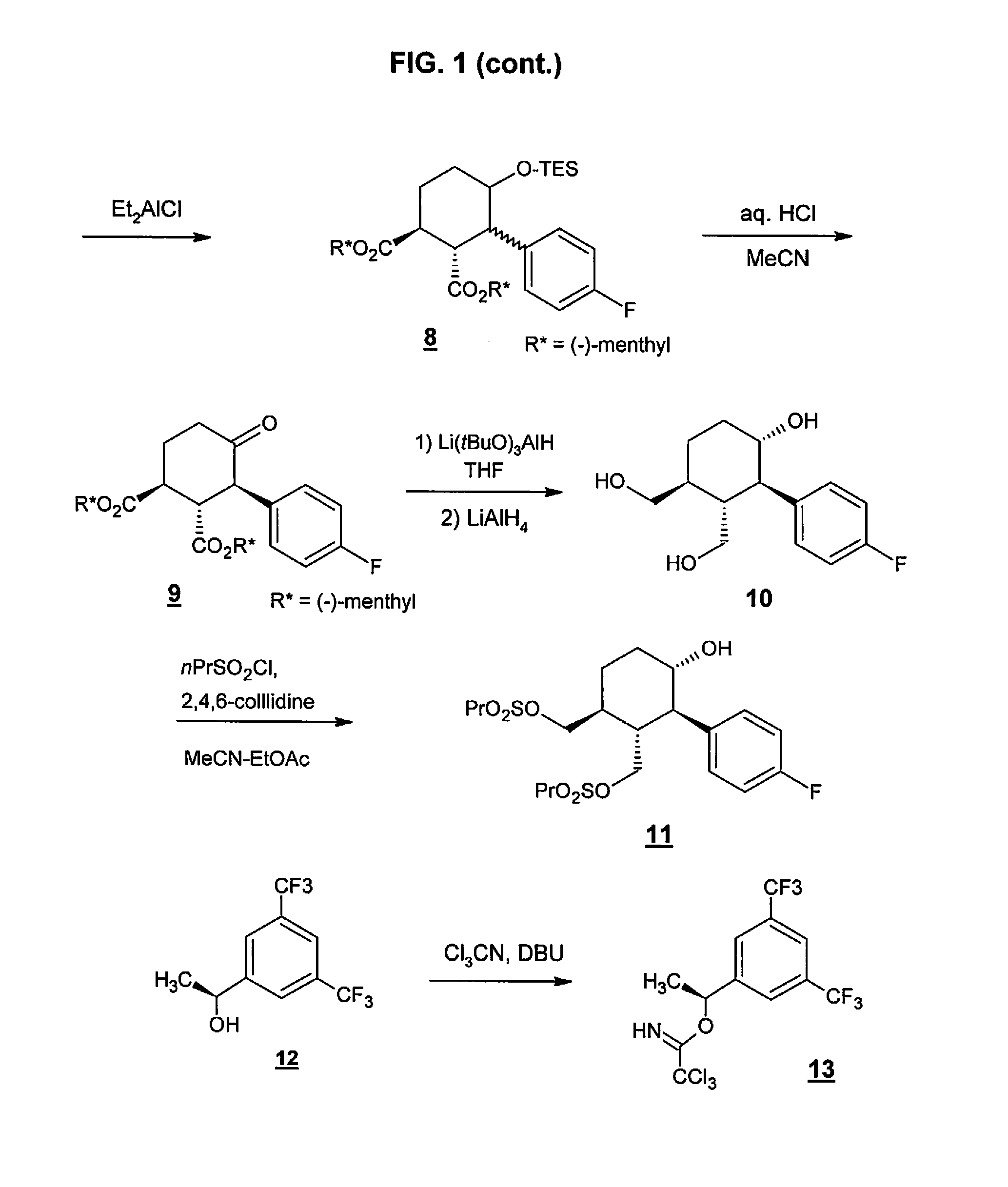
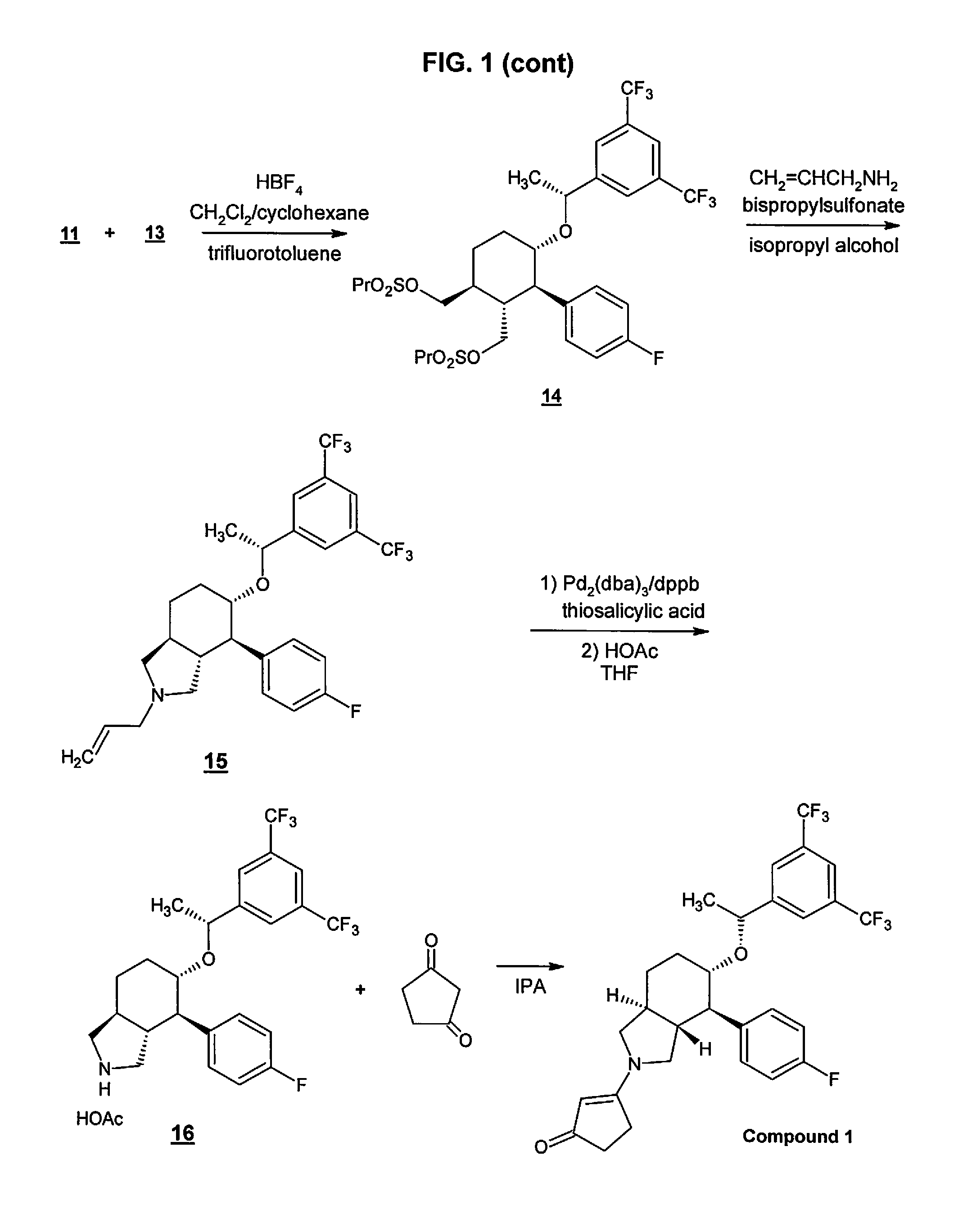
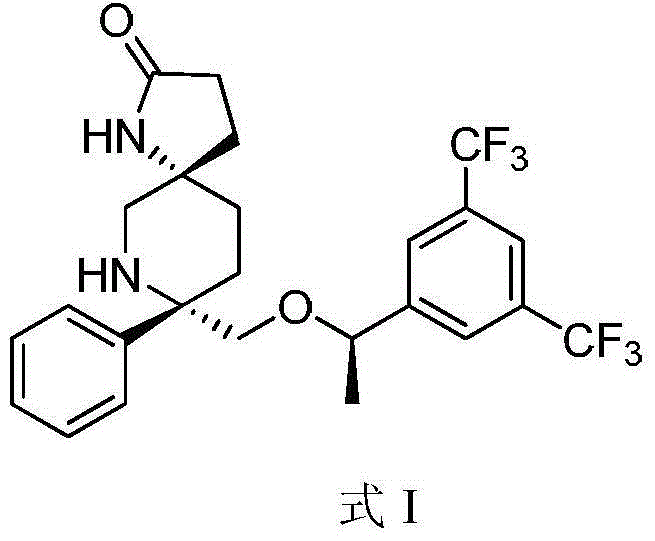
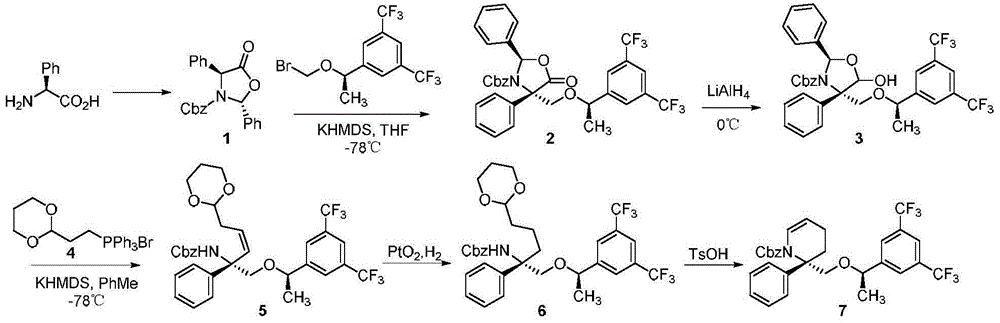
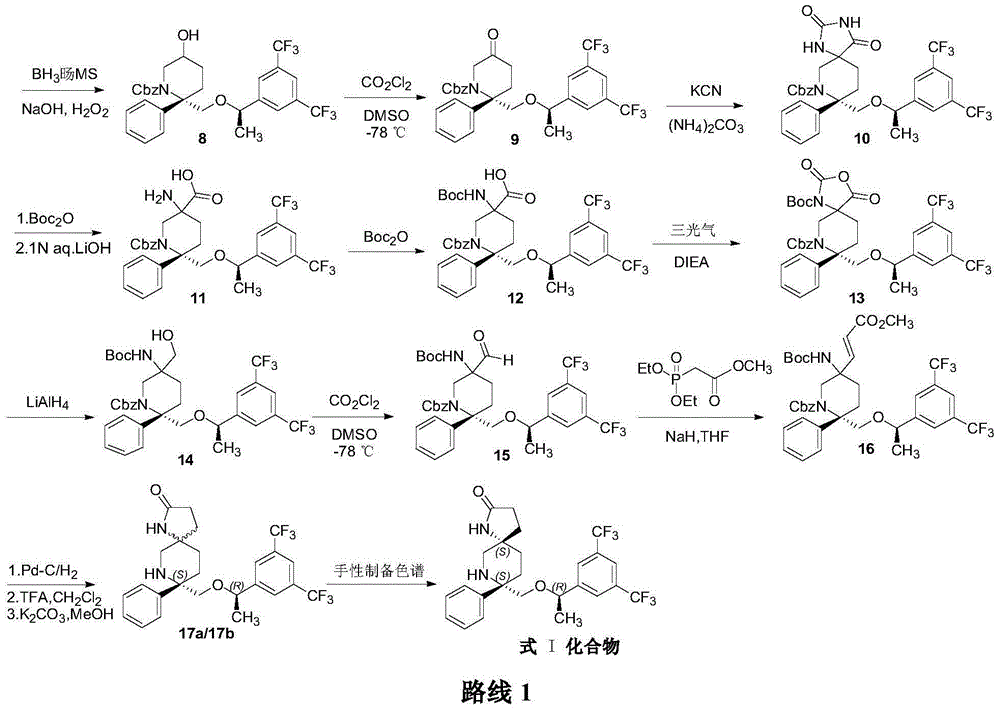
Preparation method of NK-1 receptor antagonist and intermediate thereof
ActiveCN105017251AReduce usageEasy to operateGroup 4/14 element organic compoundsPreparation from carboxylic acid halidesNK 1 Receptor AntagonistsPhenyl group
The invention relates to a preparation method of an NK-1 receptor antagonist and an intermediate thereof. The invention provides a preparation method of (5S, 8s)-8-{[1-(3, 5-bis-(trifluoromethyl)phenyl)-ethoxy]methyl}-8-phenyl-1, 7-diaza-spiro[4, 5]dec-2-one (formula I compound), which is prepared from a formula II compound, and also provides the formula II compound and a preparation method thereof. The method provided by the invention has the advantages of simple operation, mild reaction condition, high safety factor, little side reaction, high yield and high purity, lowers the production cost and operation risk, and is very suitable for large-scale industrial production. (formula II as shown in the specification).
Owner:QILU PHARMA HAINAN +1
Use of NK-1 receptor antagonists for treating hypomagnesemia, neurogenic inflammation, and cardiac dysfunction associated with EGFR-blocking drugs
ActiveUS9474761B2Effectively diminish, alleviate, or otherwise preventNegative side effectOrganic active ingredientsCardiac dysfunctionSide effect
The present disclosure provides methods for alleviating or preventing the negative physiological side effects associated with the administration of EGFR blocking therapeutics. The disclosure provides, inter alia, methods for treating or preventing: hypomagnesemia, cardiac dysfunction, and skin lesions, which are induced by EGFR blocking drugs, by administering an NK-1 receptor antagonist.
Owner:GEORGE WASHINGTON UNIVERSITY
Use of NK-1 receptor antagonist serlopitant in pruritus
Owner:VYNE THERAPEUTICS INC
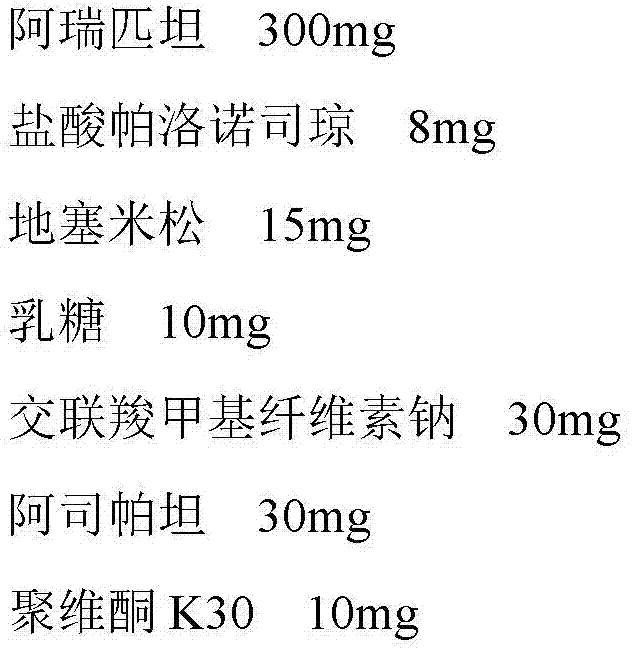
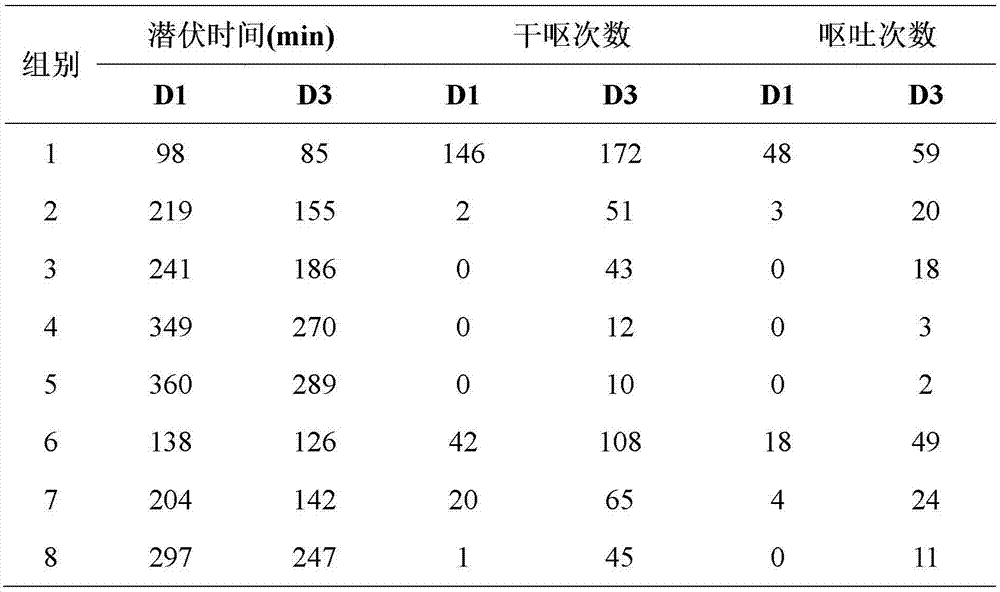
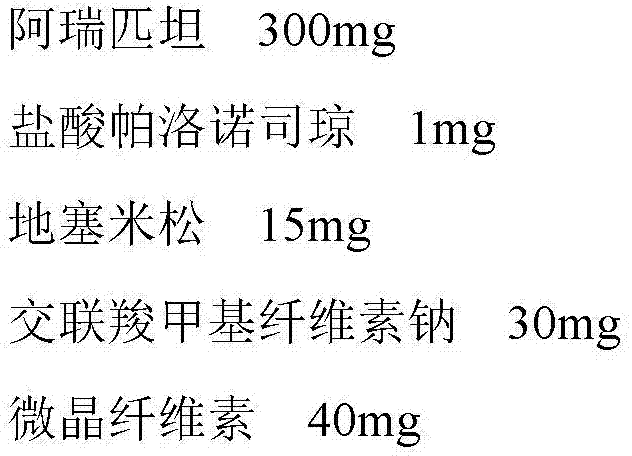
A pharmaceutical composition for treating vomiting
InactiveCN103520725BImprove complianceDigestive systemHeterocyclic compound active ingredientsAdrenal cortex hormonesPatient compliance
Owner:HAISCO PHARMA GRP INC
Genetic polymorphisms in the preprotachykinin gene
InactiveUS20060228752A1Useful predictionOrganic active ingredientsSenses disorderPreprotachykininNK 1 Receptor Antagonists
The present invention relates to a method for correlating single nucleotide polymorphisms in the preprotachykinin (NKNA) gene with the efficacy and compatibility of a pharmaceutically active compound administered to a human being. The invention further relates to a method for determining the efficacy and compatibility of a pharmaceutically active compound administered to a human being which method comprises determining at least one single nucleotide polymorphism in the NKNA gene. Said methods are based on determining specific single nucleotide polymorphisms in the NKNA gene and determining the efficacy and compatibility of a pharmaceutically active compound in the human by reference to polymorphism in NKNA. The invention further relates to isolated nucleic acids comprising within their sequence the polymorphisms as defined herein, to nucleic acid primers and oligonucleotide probes capable of hybridizing to such nucleic acids and to a diagnostic kit comprising one or more of such primers and probes for detecting a polymorphism in the NKNA gene, to a pharmaceutical pack comprising NK-1 receptor antagonists and instructions for administration of the drug to human beings tested for the polymorphisms as well as to a computer readable medium with the stored sequence information for the polymorphisms in the NKNA gene.
Owner:FOERNZLER DOROTHEE +5
NK-1 receptor antagonists for improving anesthesia recovery
The present invention is directed to the administration of a compound of the Formula (I)and (Ia), wherein R<2> is selected from the group consisting of methyl, ethyl, isopropyl, sec-butyl and tert-butyl, to an animal to improve anesthesia recovery.
Owner:PFIZER PROD INC
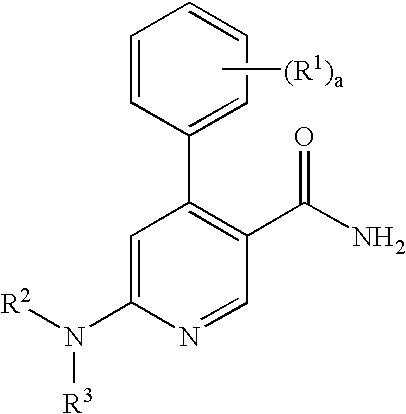
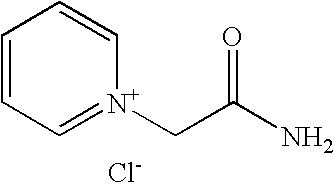
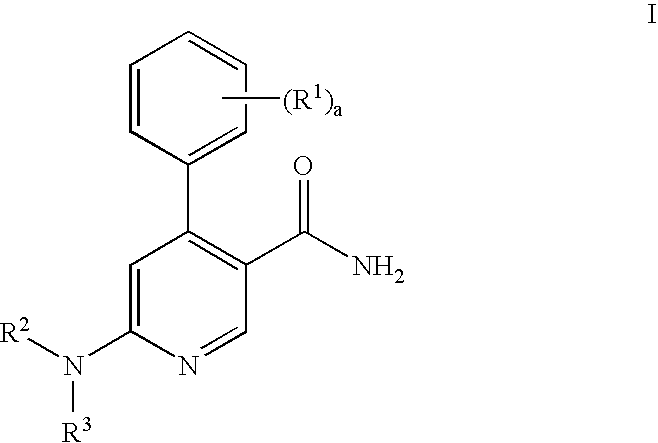
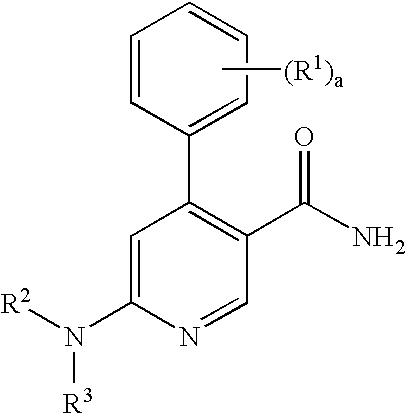
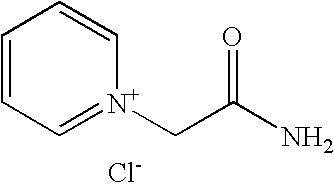
Process for preparation of pyridine derivatives of NK-1 receptor antagonist
ActiveUS7384939B2Organic chemistryPhosphorous compound active ingredientsNK1 receptor antagonistPyridine
The present invention provides a process for preparing a pyridine compound of the formula:wherein R1, R2, R3 and a are those defined herein.
Owner:ROCHE PALO ALTO LLC
Process for preparation of pyridine derivatives of NK-1 receptor antagonist
ActiveUS20060014959A1Organic chemistryHeterocyclic compound active ingredientsPyridineNK 1 Receptor Antagonists
The present invention provides a process for preparing a pyridine compound of the formula: wherein R1, R2, R3 and a are those defined herein.
Owner:ROCHE PALO ALTO LLC
Use of NK-1 receptor antagonists in management of visceral pain
Methods for managing visceral pain in mammalian subjects are described, in which a NK-1 receptor antagonist is administered to the subject before, during or after administration of general anesthesia. The methods and uses of NK-1 receptor antagonists described herein provide improved visceral pain management and MAC reduction when used with volatile anesthetics for general anesthesia.
Owner:COLORADO STATE UNIVERSITY
Method of administering emulsion formulations of an nk-1 receptor antagonist
Disclosed herein are methods of administering pharmaceutical formulations of a neurokinin-1 (NK-1) receptor antagonist to a subject in need of treatment of emesis.
Owner:HERON THERAPEUTICS
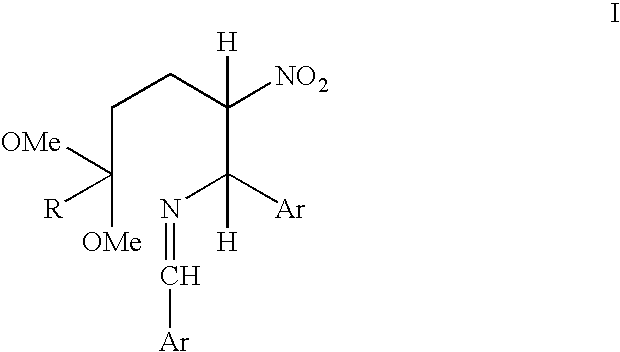
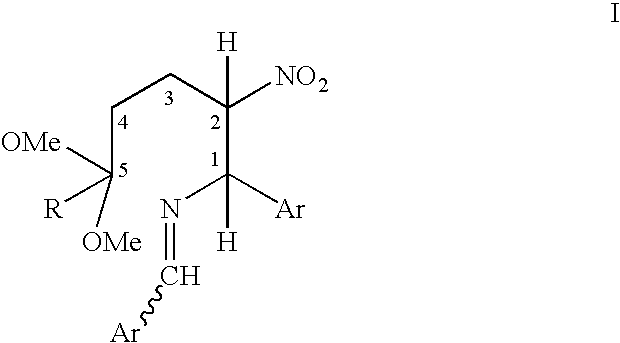
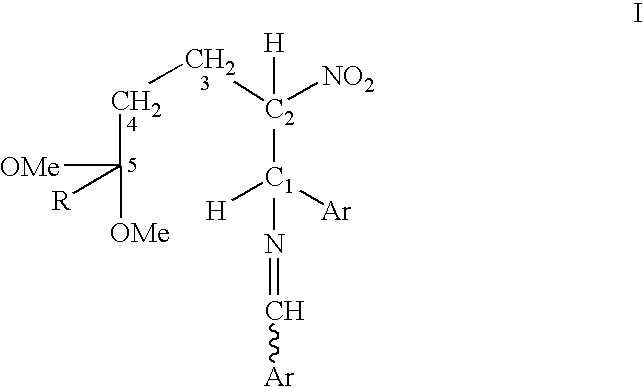
Process for converting a cis-trans mixture of substituted benzylidene amines into the pure cis isomer
InactiveUS6878848B2Optically-active compound separationImino compound preparationNK 1 Receptor AntagonistsBenzamide
A process for interconverting a mixture of cis-trans isomers of a compound of formula I into the substantially pure cis isomer. Cis isomers of formula I are useful intermediates in the synthesis of cis isomers of benzamide piperidine compounds which exhibit activity as NK-1 receptor antagonists.
Owner:PFIZER INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com