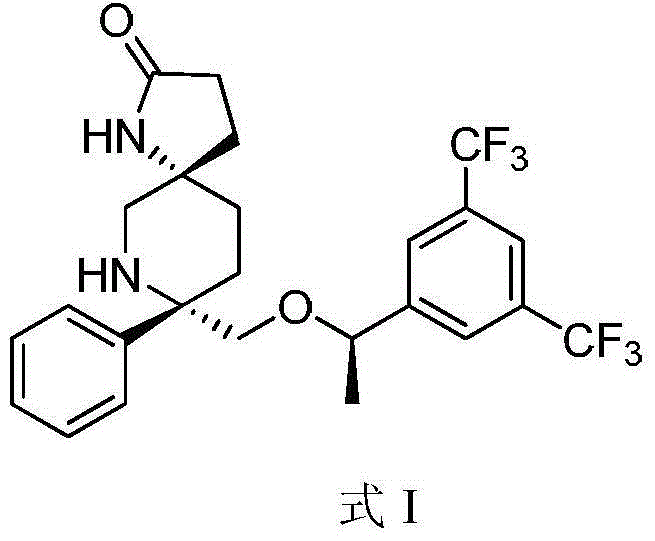
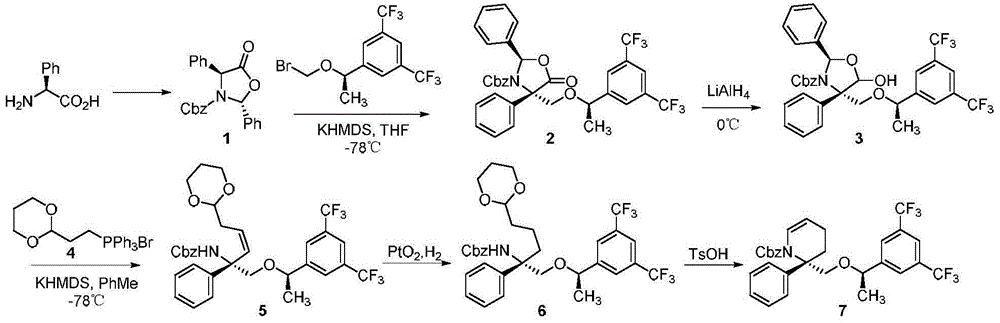
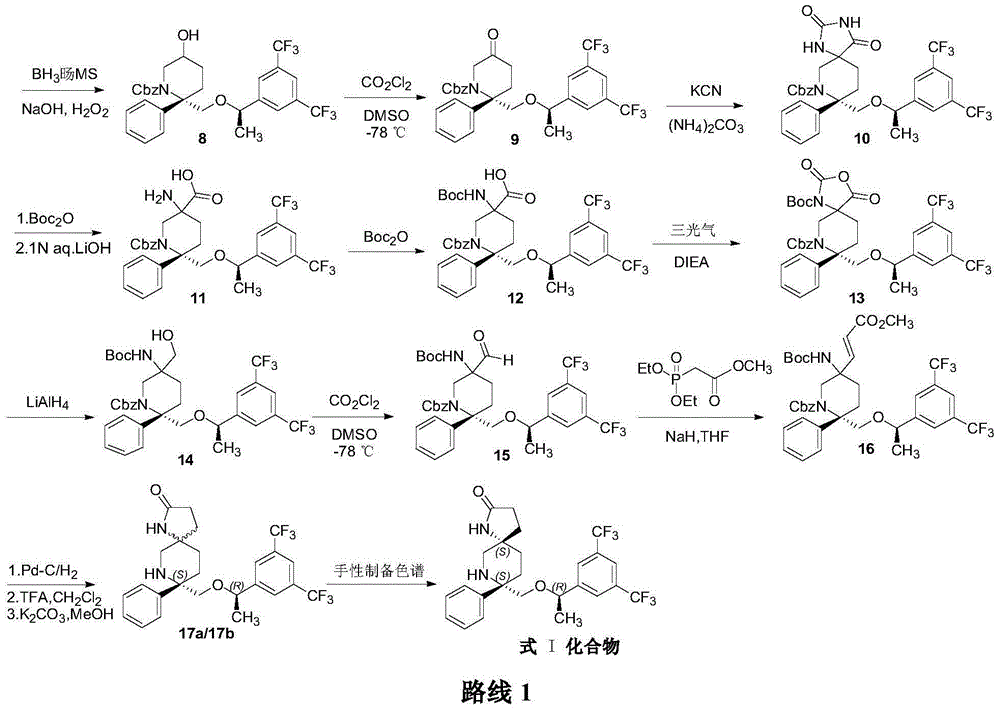
Preparation method of NK-1 receptor antagonist and intermediate thereof
A technology of -8-{, compounds, applied in the field of key intermediates and their preparation, can solve problems such as cost increase, unfavorable environmental protection, and process cost increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Example 1 Preparation of the following formula (S)-1-(tert-butyldiphenylsilyloxy)-2-phenylbut-3-en-2-amine
[0101]
[0102] Weigh 2-hydroxyacetophenone (200.0g, 1.47mol), add 2000ml of dichloromethane and tert-butyldiphenylchlorosilane (445.3g, 1.62mol) successively, and make the temperature of the mixture drop to 0-10 ℃, add triethylamine (180.0g, 1.78mol) dropwise, after the dropwise addition, react at room temperature until the conversion of 2-hydroxyacetophenone is complete, add water for extraction, separate liquids to obtain an organic phase, add anhydrous sodium sulfate to dry, dry After the completion, the desiccant was filtered off, and the filtrate was concentrated under reduced pressure to obtain an oil.
[0103] Add 5500ml of toluene to the obtained oil, and add tetraisopropyl titanate (829.7g, 2.94mol) and (R)-(+)-tert-butylsulfinamide (213.3g, 1.76mol) successively under stirring, The mixture was heated to 70-80°C to react. After the reaction was com...
Embodiment 2
[0108] Example 2 Preparation of the following formula (S)-1-benzyloxy-2-phenyl-but-3-en-2-amine
[0109]
[0110]Weigh 2-hydroxyacetophenone (150.0g, 1.10mol), add 1500ml tetrahydrofuran, stir and cool down to 0-5°C, and benzyl bromide (226.0g, 1.32mol), and stir to lower the temperature of the mixture to 0-5°C. 10°C, add sodium tert-butoxide (126.9g, 1.32mol), dropwise addition is complete, react until the conversion of 2-hydroxyacetophenone is complete, add 200ml of water, concentrate under reduced pressure to remove tetrahydrofuran, add 1000ml of dichloromethane for extraction, and separate The obtained organic phase was dried by adding anhydrous sodium sulfate. After drying, the desiccant was filtered off, and the filtrate was concentrated under reduced pressure to obtain an oily substance.
[0111] Add 2000ml of toluene to the obtained oil, and add tetraisopropyl titanate (622.3g, 2.21mol) and (R)-(+)-tert-butylsulfinamide (200.4g, 1.65mol) successively under stirring,...
Embodiment 3
[0116] Example 3 Preparation of the following formula (S)-1-(4-chlorobenzoyl)oxy-2-phenyl-but-3-en-2-amine
[0117]
[0118] Weigh (R)-N-[(S)-1-(tert-butyldiphenylsilyloxy)-2-phenyl-but-3-en-2-yl prepared according to the method of Example 1 ]-2-methylpropyl-2-sulfenamide (10.1g, 0.02mol), add 100ml tetrahydrofuran, add tetrabutylammonium fluoride (10.5g, 0.04mol), stir the reaction, after the reaction, add water to extract , separated the organic phase, dried over anhydrous sodium sulfate, filtered off the desiccant after drying, cooled the filtrate to 0-5°C, added triethylamine (4.0g, 0.04mol), dropped p-chlorobenzoyl chloride (4.2 g, 0.024mol), after the reaction is complete, add purified water to extract, separate liquids, after the organic phase is dried over anhydrous sodium sulfate, filter the desiccant, and concentrate the filtrate under reduced pressure to obtain (R)-N-[(S) -1-(4-Chlorobenzoyloxy)-2-phenyl-but-3-en-2-yl]-2-methylpropyl-2-sulfinamide 6.33 g, yield ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com