Use of NK-1 receptor antagonist serlopitant in pruritus
A dosage and cream technology, applied in the direction of medical preparations containing active ingredients, pill delivery, drug combination, etc., can solve problems such as lack of clinical experience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0175] Example 1. Preparation of Serlopitant Tablets
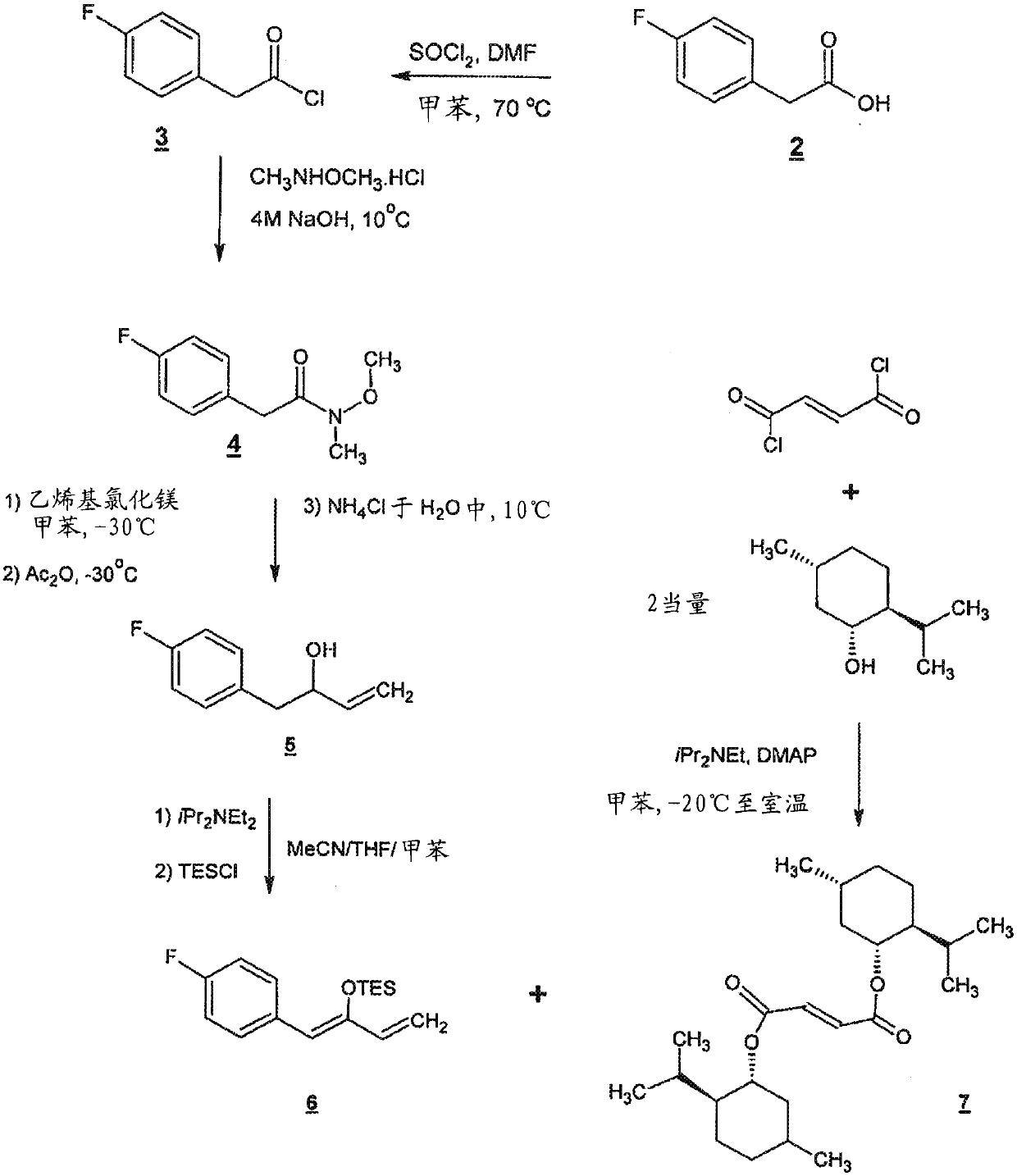
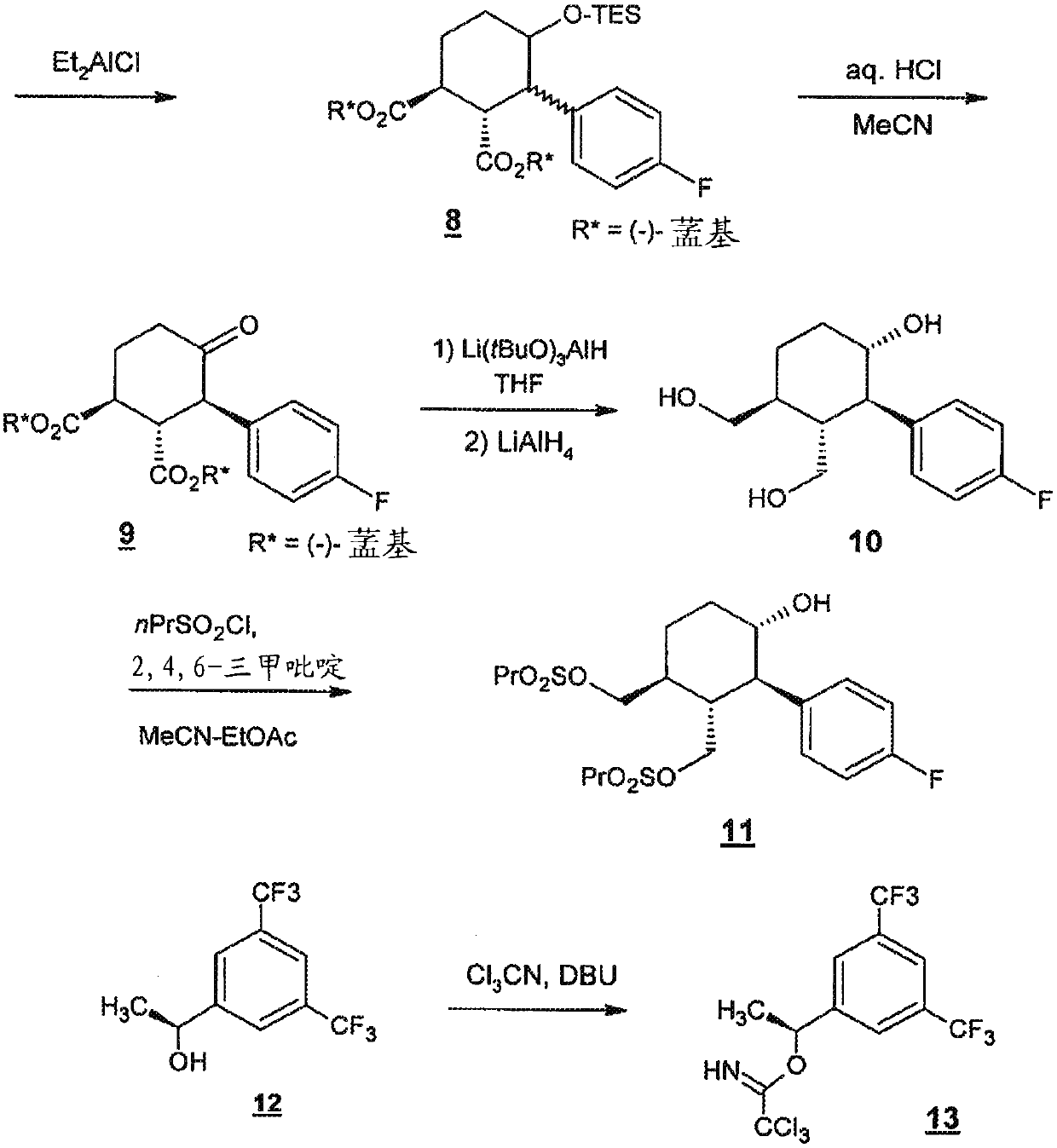
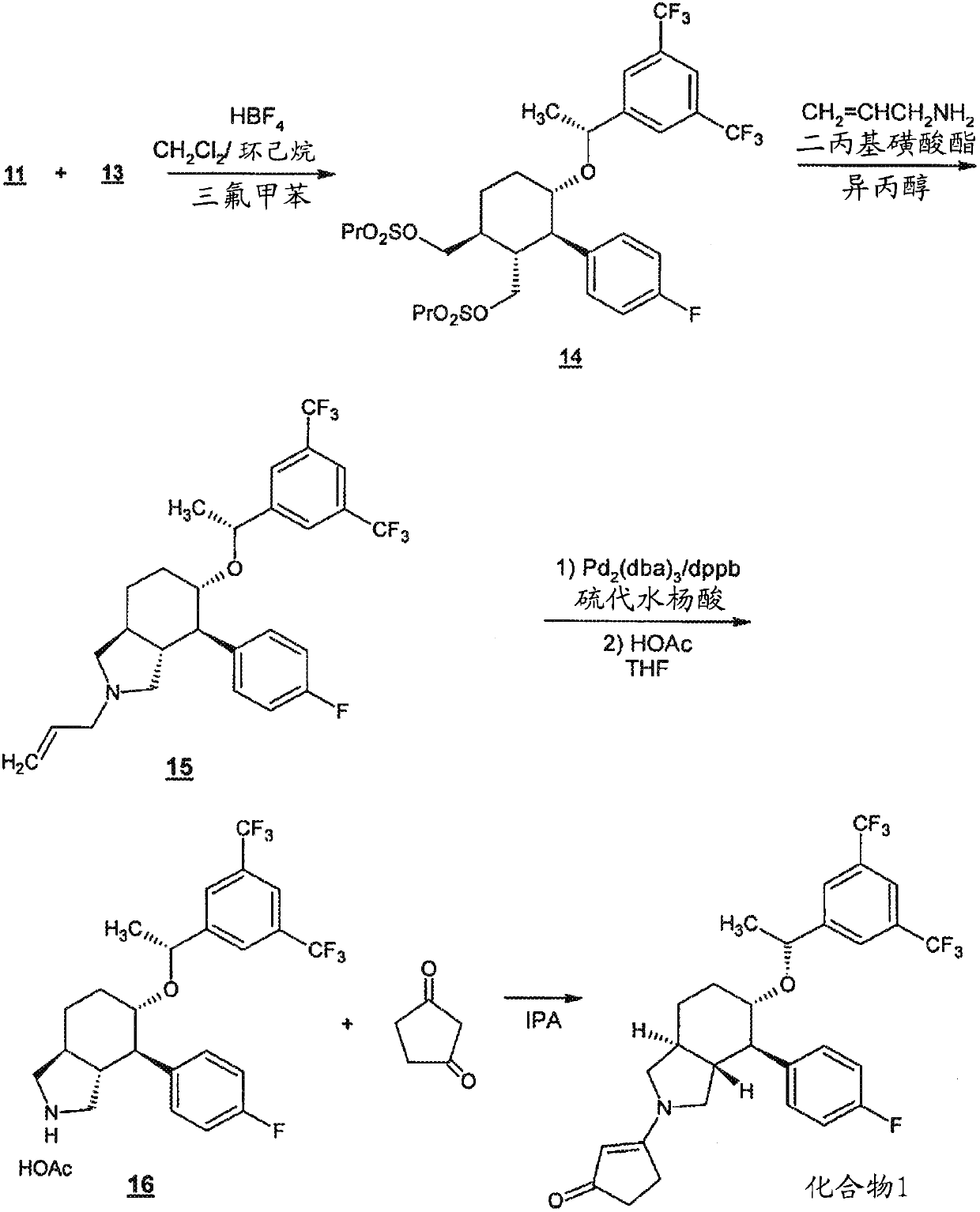
[0176] Serlopitant, 3-[(3aR,4R,5S,7aS)-5-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-4-( 4-fluorophenyl)-1,3,3a,4,5,6,7,7a-octahydroisoindol-2-yl]cyclopent-2-en-1-one (Compound 1) can be formulated In the form of tablets for oral use. Table 1 shows the qualitative / quantitative composition of exemplary doses. The amount of excipients can vary slightly (+ / - 10%) during drug development.
[0177] Table 1
[0178]
[0179]Tablet strengths of 0.25, 1 and 5 mg were prepared as compressed tablet formulations. The tablet manufacturing method was the same for all proposed potencies. The method consists of the following steps: 1) mixing compound 1, mannitol and sodium lauryl sulfate; 2) adding the remaining mannitol into a blender and mixing; 3) mixing microcrystalline cellulose, croscarmellose Sodium and colloidal silicon dioxide are added to the mixer containing the above mixture to complete the mixing and de-agglom...
Embodiment 2
[0180] Example 2. Preparation of Serlopitant Capsules
[0181] Serlopitant (Compound 1) is also supplied to clinics as liquid-filled capsules. Table 2 shows the qualitative / quantitative composition of exemplary doses. The amount of excipients can vary slightly (+ / - 10%) during drug development.
[0182] Table 2
[0183]
[0184] *Capsules are supplied by Capsugel (Morristown, NJ) and contain gelatin and titanium dioxide
[0185] ** Approximate weight of empty capsule shell
[0186] ***Required to seal the capsule shell
[0187] The formulations are prepared by dissolving the drug in mono- and diglycerides. In addition, 0.1% by weight of butylated hydroxyanisole was added as an antioxidant. The initial capsule strength was dispensed into hard gelatin capsules and sealed by spraying with a 1:1 (w / w) water:ethanol solution. Subsequent potencies (including 0.25, 1 and 4 mg) were dispensed into hard gelatin capsules and sealed with gelatin / polysorbate 80 strips. Corres...
Embodiment 3
[0189] Example 3. Clinical Study of Serlopitant in Chronic Pruritus
[0190] The good feasibility of testing the efficacy of three doses of serlopitant in the treatment of chronic pruritus was performed according to the ICH's guidelines for good clinical practice, US federal regulations, the Health Insurance Portability and Accountability Act (HIPAA), and any local regulatory requirements. controlled human clinical trials. The study is a phase II randomized, double-blind, parallel-group, placebo-controlled, multicenter trial designed to test the efficacy and safety of several doses of serlopitant relative to placebo in patients with chronic pruritus sex. The patient population for the study included adults, male or female, 18 to 72 years old. The patient must have been previously diagnosed with chronic pruritus defined as pruritus greater than 6 weeks and a VAS score greater than 7 due to any etiology (except uremia, liver failure, cancer or cancer treatment).
[0191] Pa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com