Use of nk-1 receptor antagonists in pruritus
a technology of nk-1 receptor and antagonist, which is applied in the direction of drug compositions, biocide, heterocyclic compound active ingredients, etc., can solve the problems of severe debilitating, adversely affecting the quality of life, and limited solid epidemiological data
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Serlopitant Tablets
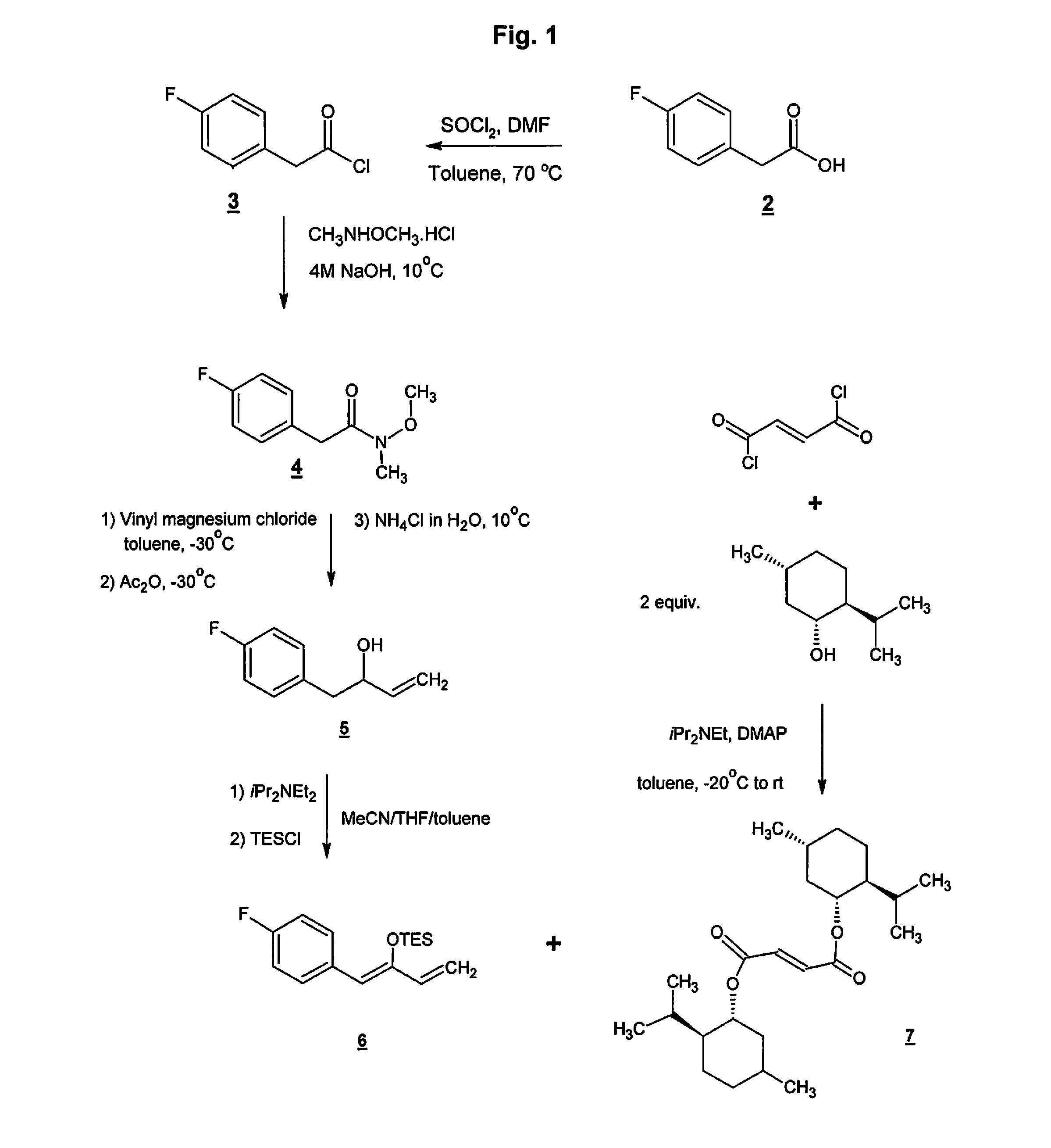
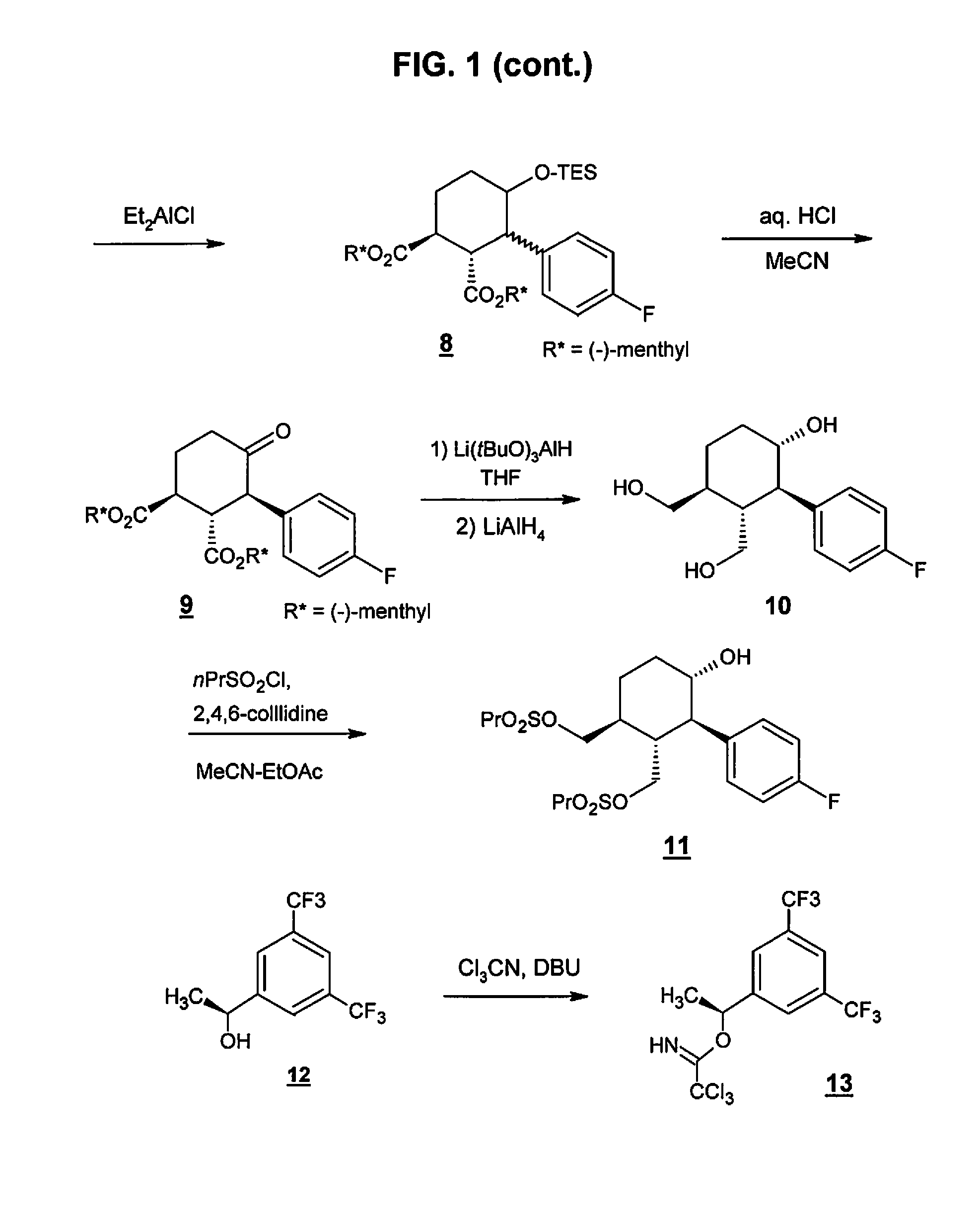
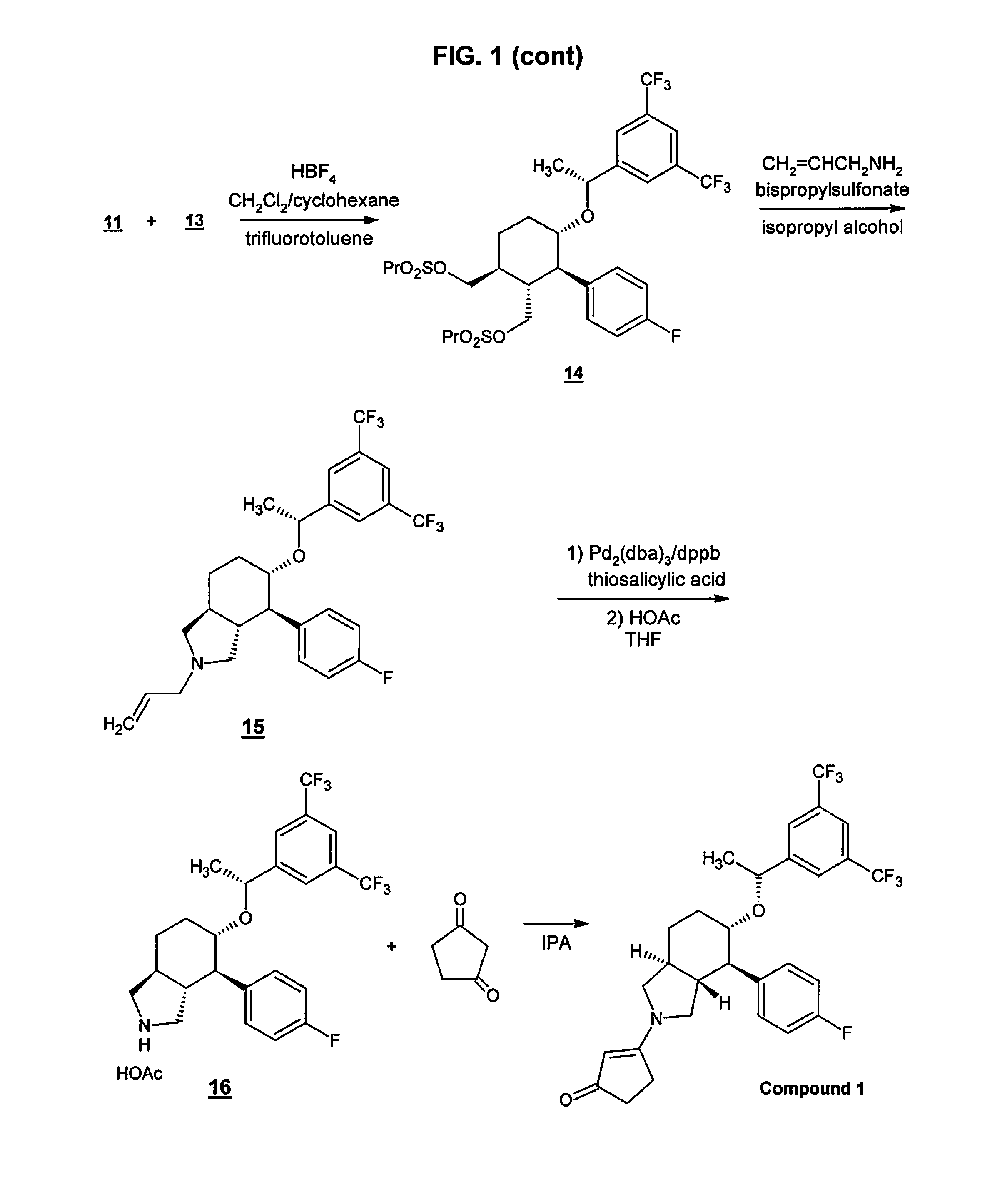
[0065]Serlopitant, 3-[(3aR,4R,5S,7aS)-5-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-4-(4-fluorophenyl)-1,3,3a,4,5,6,7,7a-octahydroisoindol-2-yl]cyclopent-2-en-1-one, Compound 1, may be formulated as a tablet for oral use. Table 1 shows the qualitative / quantitative composition of exemplary dosages. Minor variations in the excipient quantities (+ / −10%) may occur during the drug development process.
TABLE 1ComponentsFunction% of compositionCompound 1Active agent1-6%Microcrystalline celluloseDiluent50-60% MannitolDiluent20-30% Croscarmellose SodiumDisintegrant1-3%Colloidal silicaDisintegrant0.25-0.5% Sodium Lauryl SulfateSurfactant5-6%Magnesium StearateLubricant0.25-2% Total Tablet Composition100%
[0066]Tablet potencies of 0.25, 1 and 5 mg are prepared as a compressed tablet formulation. The tablet manufacturing process is the same for all proposed potencies. The process consists of the following steps: 1) Compound 1, mannitol and sodium lauryl sul...
example 2
Preparation of Serlopitant Capsules
[0067]Serlopitant (Compound 1) may also be supplied to the clinic as liquid-filled capsules. Table 2 shows the qualitative / quantitative composition of exemplary dosages. Minor variations in the excipient quantities (+ / −10%) may occur during the drug development process.
TABLE 2Unit StrengthComponentsFunction0.25 mg1 mg4 mgCapsule FillCompound 1Active agent0.25 mg 1 mg 4 mgMono- & Di-glyceridesSolubilizer 399 mg398.6 mg 395.6 mg Butylated HydroxyanisoleAntioxidant0.40 mg0.40 mg0.40 mgCapsule Shell#0 White Opaque Hard GelatinCapsule shell 96 mg** 96 mg** 96 mg**Capsule*Gelatin***Banding———componentPolysorbate 80***Banding———component*Capsules are provided by Capsugel (Morristown, NJ) and contain gelatin and titanium dioxide**Approximate weight of empty capsule shell***As needed to seal the capsule shells
[0068]The formulation is prepared by dissolving the drug substance in mono- and di-glycerides. Furthermore, 0.1 wt % butylated hydroxyanisole ...
example 3
Clinical Study of Serlopitant in Chronic Pruritus
[0070]A well-controlled human clinical trial testing the efficacy of three dosages of serlopitant in the treatment of chronic pruritus is conducted in accordance with the ICH Guidelines for Good Clinical Practices, the U.S. Code of Federal Regulations, the Health Insurance Portability and Accountability Act (HIPAA), and any local regulatory requirements. The study is a Phase II randomized, double-blind, parallel group, placebo-controlled, multicenter trial designed to test the efficacy and safety of several doses of serlopitant versus placebo in patients with chronic pruritus. The study patient population includes adult, males or females, 18 to 72 years of age. The patients must be previously diagnosed with chronic pruritus caused by any etiology, except uremia, hepatic failure, cancer or cancer therapy, with chronic pruritus defined as greater than 6 weeks of itching and a VAS score of greater than 7.
[0071]Patients are randomized to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com