Azaphenanthrene fluorene derivatives, preparation method and electroluminescence light-emitting device
A technology of fluorene derivatives and azophenanthrene, which is applied in the field of organic light-emitting materials, can solve the problems of device instability and low luminous efficiency, and achieve the effects of improving device efficiency, reducing red shift, and increasing glass transition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
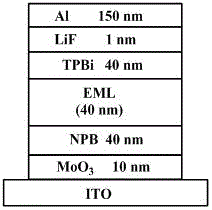
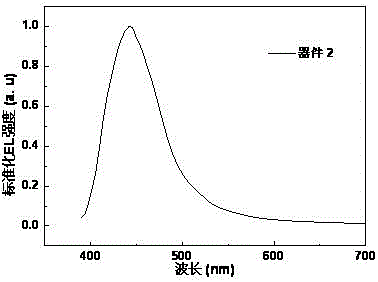
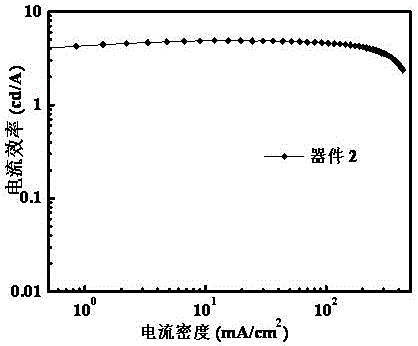
[0032] The present invention provides an azaphenanthrene fluorene derivative, a preparation method and an electroluminescent light-emitting device. In order to make the purpose, technical solution and effect of the present invention clearer and more definite, the present invention will be further described in detail below. It should be understood that the specific embodiments described here are only used to explain the present invention, not to limit the present invention.
[0033] This embodiment provides azaphenanthrene fluorene derivatives, the general structural formula of which is as follows:
[0034] , where Ar 1 and Ar 2 Both are carbazole groups.
[0035] Among them, the Ar 1 and Ar 2 One of the following combinations:
[0036] Ar 1 =Ar 2 = , at this time, the azaphenanthrene fluorene derivatives are: 9,9'-diphenyl-azaphenanthrene fluorene (abbreviated as DPPhF);
[0037] Ar 1 = , Ar 2 = , at this time, the azaphenanthrene fluorene derivatives are: 9-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com