High rate, long cycle life battery electrode materials with an open framework structure
A technology of electrode materials and anode materials, applied in battery electrodes, structural parts, primary batteries, etc., can solve problems such as reducing round-trip energy efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
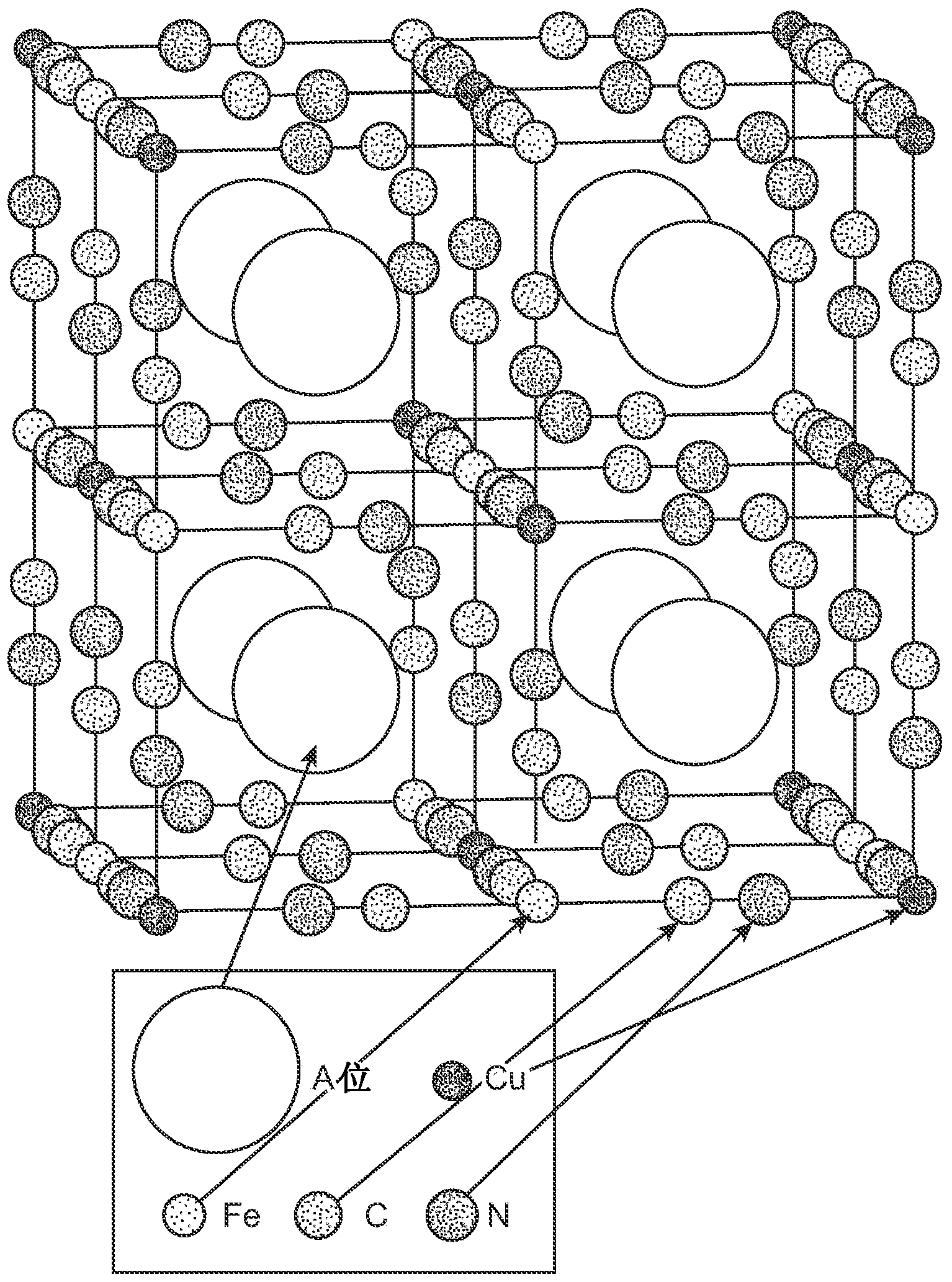
[0099] copper hexacyanoferrate
[0100] This example describes copper hexacyanoferrate ("CuHCF"), whose electrochemical reaction can be expressed as KCuFe III (CN) 6 +xK + +xe - =K 1+x Cu[Fe II (EN) 6 ] x [Fe III (EN) 6 ] 1-x . Anhydrous KCuFe(CN) 6 The theoretical specific capacity for some embodiments is about 85 mAh / g. In practice and for some embodiments, capacities of about 60 mAh / g and lower are typically observed because the framework structure contains zeolite water. Inductively coupled plasma mass spectrometry of CuHCF synthesized by the bulk precipitation reaction found a K:Cu:Fe ratio of approximately 0.71:1:0.72. Following the previous convention for hydration of crystal structures, the formula for copper-based materials is K 0.71 Cu[Fe(CN) 6 ] 0.72 3.7H 2 O. The theoretical specific capacity of the material with this formula is about 62 mAh / g, which is consistent with the observed specific capacity. Because the exact water content varies with te...
Embodiment 2
[0112] Nickel hexacyanoferrate
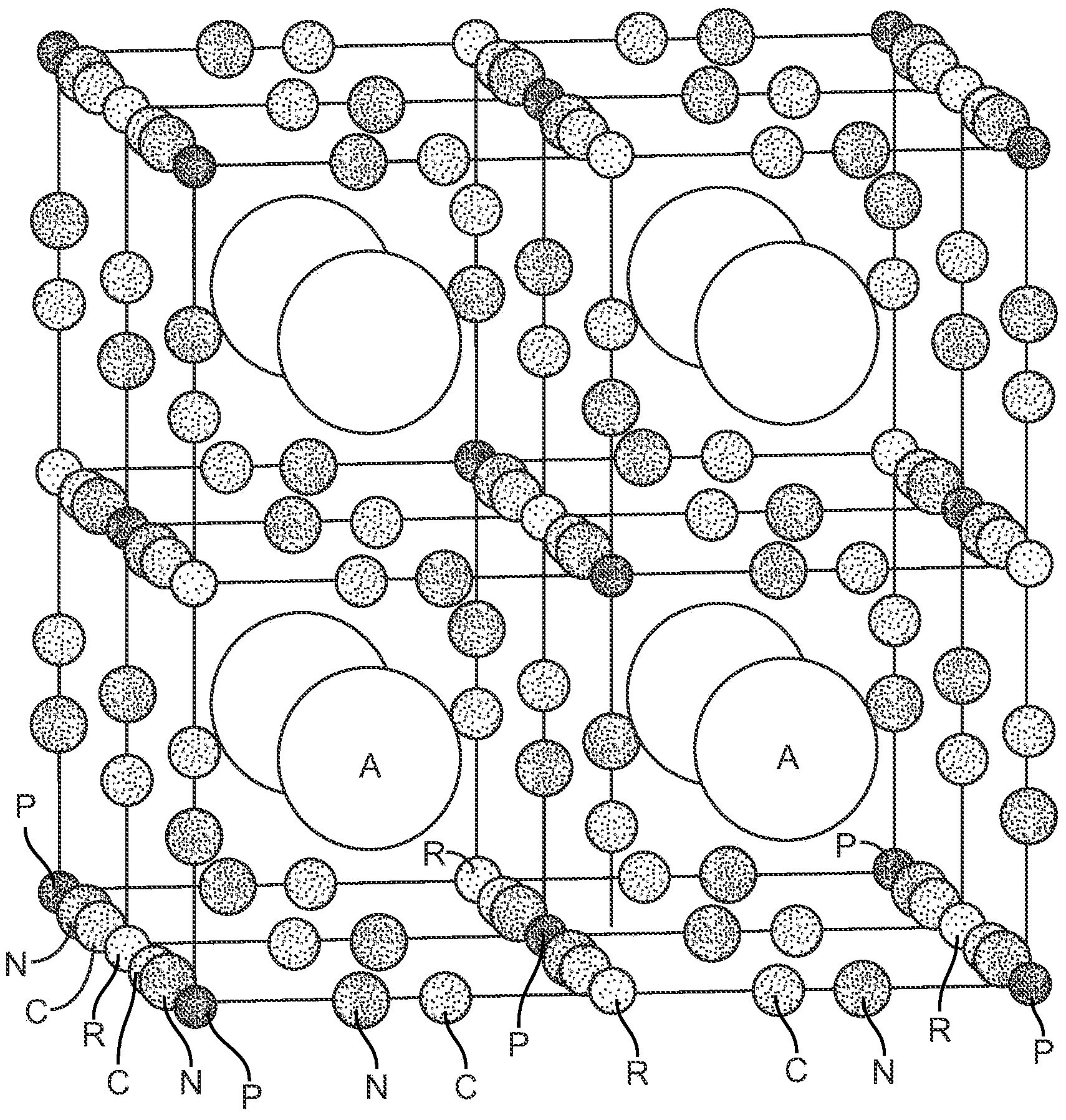
[0113] This example describes nickel hexacyanoferrate ("NiHCF"), whose electrochemical reaction can be expressed as ANiFe III (EN) 6 +A + +e – =A 2 NiFe II (EN) 6 , where A + is a cation such as sodium or potassium. The following describes the unique behavior of high-capacity battery electrodes comprising bulk NiHCF powders prepared by the chemical precipitation method.
[0114] NiHCF has a Prussian blue crystal structure in which transition metal cations such as Fe and Ni are bound by bridging CN ligands to form a face-centered cubic structure (see Image 6 ). In the case of NiHCF, Fe is sixfold carbon coordinated, while Ni (e.g., Ni 2+ ) is six heavy nitrogen coordination. The resulting framework has large channels oriented in the direction, and hydrated cations such as K + and Na + can diffuse through it. These cations occupy interstitial "A" sites on the center of each of the eight subcells of the unit cell. When the materia...
Embodiment 3
[0125] Copper hexacyanoferrate and nickel hexacyanoferrate
[0126] This example describes additional measurements of CuHCF and NiHCF of some embodiments. To investigate the effect of intercalation species on the electrochemical properties of bulk CuHCF and NiHCF, these materials were cycled in aqueous electrolytes containing lithium, sodium, potassium, or ammonium ions.
[0127] The synthesis of CuHCF and NiHCF nanopowders was carried out by co-precipitation method. Simultaneous dropwise addition of approximately 40 mM copper or nickel nitrate and approximately 20 mM potassium hexacyanoferrate to deionized water allowed controlled co-precipitation of solid CuHCF or NiHCF product. The synthesis of CuHCF is carried out at room temperature, while the synthesis of NiHCF is carried out at about 70°C. These solid products were filtered, washed with water, and dried under vacuum at room temperature. Up to about 3 g of product are produced during each synthesis, and these synthese...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com