Acyl-coenzyme A synthetase and application thereof
A technology for synthesizing enzymes and coenzymes, applied in the field of enzyme engineering, can solve the problems that the biochemical functions of proteins have not yet been identified
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Construction and expression of embodiment 1 Indian ghost pepper ACS1 genetically engineered bacteria
[0037] Using ACS1-sumo-F and ACS1-sumo-R primers to amplify the ACS1 gene of the cDNA of the green fruit of the Indian ghost pepper, the sequences of the above two primers are: CGC GAA CAG ATT GGA GGT GCAACAGATAAATTTATTATTG and GTG GCG GCC GCT CTA TTA TCACTTGGTACCCTTGTACAT . PCR amplification products were purified with 1% agarose gel and mixed with linearized pETite N-His SUMO Kan expression vector (Lucigen, Middleton, WI). The DNA mixture was transformed into E. coli HI-control10G cells (Lucigen) by heat shock method. Sequence the inserted gene to ensure its sequence is correct. The encoded amino acid sequence is shown in SEQ ID NO.1. The pETiet N-His SUMO-Pipano pepper ACS1 gene was transformed into HI-Control BL21(DE31) cells (Lucigen), and the expression of His-SUMO-ACS1 was induced with 0.5mM IPTG at 16°C for 20 hours. Mixed proteins were purified by Ni-NTA c...
Embodiment 2A
[0038] Example 2 ACS1 Activity Determination
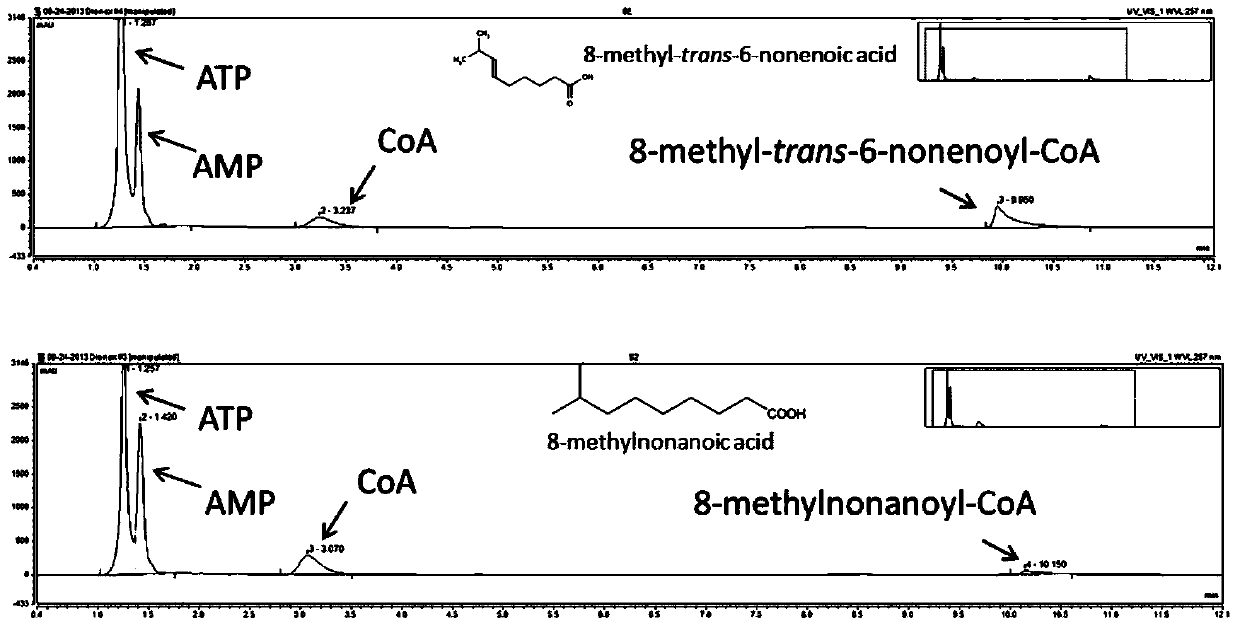
[0039] The activity of ACS1 in ghost pepper was determined by HPLC (Chen et al., 2011). Specifically, the reaction mixture (400 μL) included 0.1 M Tris-HCl, pH 7.5, 2 mM DTT, 5 mM ATP, 10 mM MgCl2, 0.5 mM CoA, 0.1% trinitrotoluene and 200 μM carboxylic acid. The reaction was initiated by adding 20 μL of purified enzyme and terminated by adding 20 μL of acetic acid after 30 minutes of reaction. HPLC use 3000LC system (Thermo Scientific), using 120C18 reverse-phase chromatographic column (Thermo Scientific; 3μ, 150x3mm). The mobile phase consisted of solvent A (0.1% trifluoroacetic acid) and solvent B (acetonitrile). The gradient elution program was as follows: 0 to 5 minutes, 5% solvent B; 5 to 9 minutes, linearly increasing solvent B concentration from 5% to 80%; 9 to 11 minutes, 80% solvent B concentration; 11 to 12 minutes, Solvent B concentration 5%. The flow rate was 0.6 mL / min. The detection range of the diode arra...
Embodiment 3
[0040] The selection of embodiment 3ACS action substrate
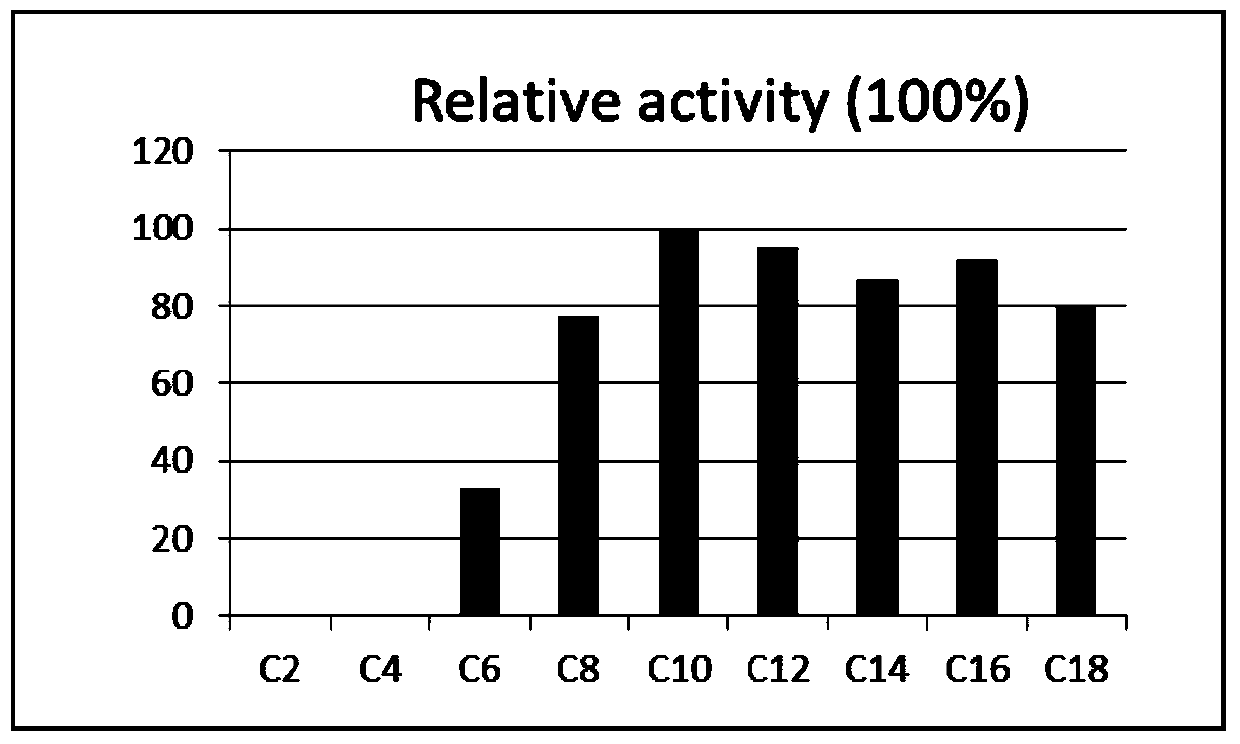
[0041] Add 5 mM acetic acid, butyric acid, caproic acid, caprylic acid, capric acid, lauric acid, myristic acid, palmitic acid and stearic acid to the enzyme reaction system respectively.
[0042] Such as figure 2 As shown, among the different substrates, capric acid is the highest activity of Indian pepper ACS1. In contrast, ACS1 had no activity against acetate and butyrate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com