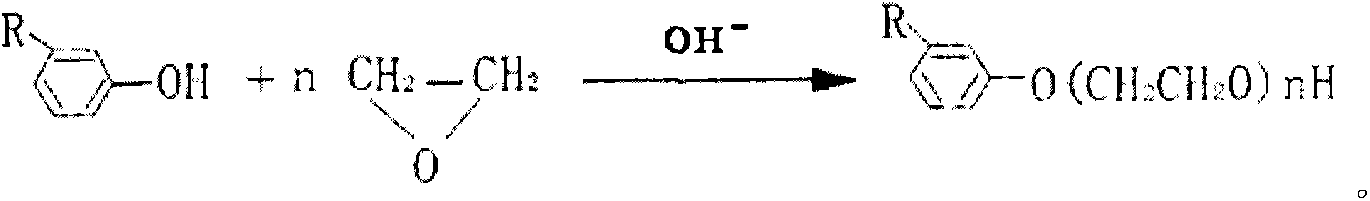High-stability disinfectant having high resistance to high temperature and high pressure
A high-stability, disinfectant technology, applied in the direction of disinfectants, waste disinfection or sterilization methods, biocides, etc., can solve the limited use of o-phthalaldehyde, poor sterilization effect and stability, and the degree of fungicide High stability, enhanced bactericidal ability and decontamination ability, and enhanced bactericidal ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1. The configuration of disinfectant:
[0042] (1) Component A: add 10.0g o-phthalaldehyde to 50.0g medical alcohol, stir to dissolve; B component: add 10.0g hydroxyquinoline to 50.0g medical alcohol, stir to dissolve; C component: Add 200.0g of cardanol to 200.0g of medical alcohol, stir to dissolve, then slowly add 20.0g of sodium hydroxide with a weight concentration of 40% under stirring, and stir to dissolve; (2) Mix component A with an appropriate amount of component C , Mix component B with the remainder of component C, then mix the above two parts of the solution, then add 80.0 g of polyethylene glycol and 380.0 g of medical alcohol, stir thoroughly to dissolve, and prepare 1000 g of the antibacterial agent.
Embodiment 2
[0043] Embodiment 2. The configuration of disinfectant:
[0044] (1) Component A: add 4.0g o-phthalaldehyde to 20.0g medical alcohol, stir to dissolve; B component: add 3.0g hydroxyquinoline to 20.0g medical alcohol, stir to dissolve; C component: Add 100.0g of cardanol to 100.0g of medical alcohol, stir to dissolve, then slowly add 10.0g of sodium hydroxide with a weight concentration of 40% under stirring, and stir to dissolve; (2) Mix component A with an appropriate amount of component C , Mix component B with the remaining component C, then mix the above two parts of the solution, then add 50.0g of polyethylene glycol and 693.0g of medical alcohol, and stir thoroughly to obtain 1000g of disinfectant.
Embodiment 3
[0045] Embodiment 3. Stability test of disinfectant:
[0046] According to the formula of Example 1, the ethanol of equal mass is used to replace the cardanol therein, and the comparison disinfectant is configured, which is hereinafter referred to as disinfectant 3. The disinfectants in Examples 1 and 2 are respectively referred to as disinfectant 1 and disinfectant 2. In the present embodiment, for the determination of o-phthalaldehyde, refer to the method of literature ("capillary gas chromatography is used to measure o-phthalaldehyde in disinfection products", the 23rd phase of Chinese Disinfection Science Impurity Volume 2006, the method of the 308-310 page) To measure.
[0047] Put 5mL of each disinfectant 1 and disinfectant 3 into sealed glass bottles, in triplicate x 4, store them in incubators at 37°C and 54°C for 14 days and 90 days respectively, then take them out and measure them respectively The content of phthalaldehyde is 100% with the content of phthalaldehyde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com