Arylnaphthyl lignans as anti-HIV agents
A technology of medicine and alkyl, applied in the field of derivatives of aryl naphthalene lignans, can solve the problem of not containing sugar units and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
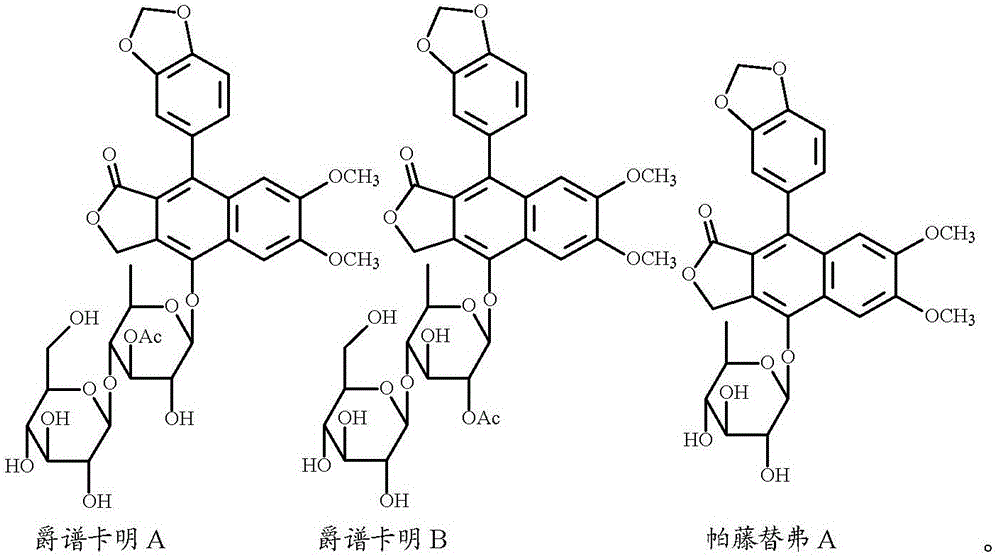
[0144] Rhizome samples of Acucus spp. were collected from Cuc Phuong National Park (Nho Quan District, Ninh Binh Province, Vietnam). Its methanol extract exhibited potent inhibitory activity against HIV replication, and IC 50 The value is 0.04 μg / mL. A 4.0 kg dry bark sample of this plant was therefore recollected from a Vietnamese National Park to identify anti-HIV compounds. Thus, two novel (1 and 2) and one known (3) arylnaphthyl lignans were isolated from this plant by bioactivity-guided fractionation studies. In structures 1 and 2, Ac stands for acetyl (-COCH 3 ), and Glu represents β-D-glucopyranosyl.
[0145]
[0146] Another sample (4.0 kg) of the rhizome of Scutellaria baicalensis was taken using MeOH extraction to provide an extract (155 g). Isolation of two novel arylnaphthalenoid lignans, japonicamin, by bioactivity-guided fractionation of MeOH extracts by column chromatography on silica gel and RP-18 reversed-phase silica gel followed by preparative HPLC se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com