Method for preparing high purity aragonite type nano calcium carbonate by acidolysis of limestones
A nano-calcium carbonate and limestone technology, applied in the direction of calcium carbonate/strontium/barium, nanotechnology, nanotechnology, etc., can solve the problems of difficulty in industrialization, high cost, large consumption, etc., and solve the problem of low utilization rate of ore resources and low cost Low, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
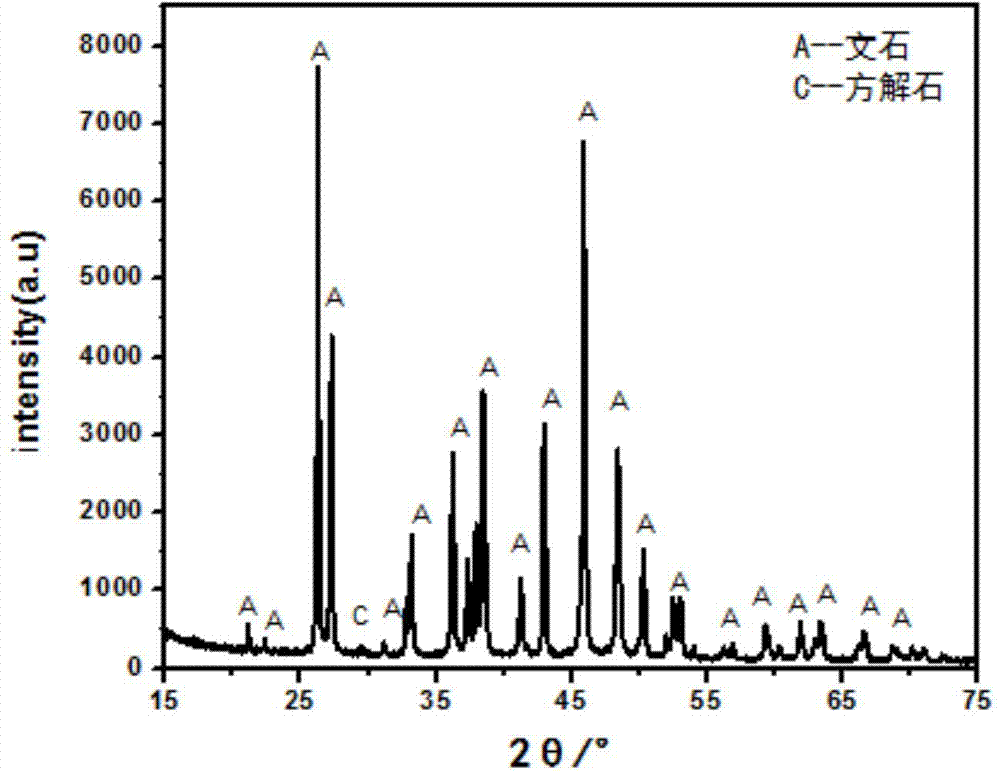
Examples
Embodiment 1
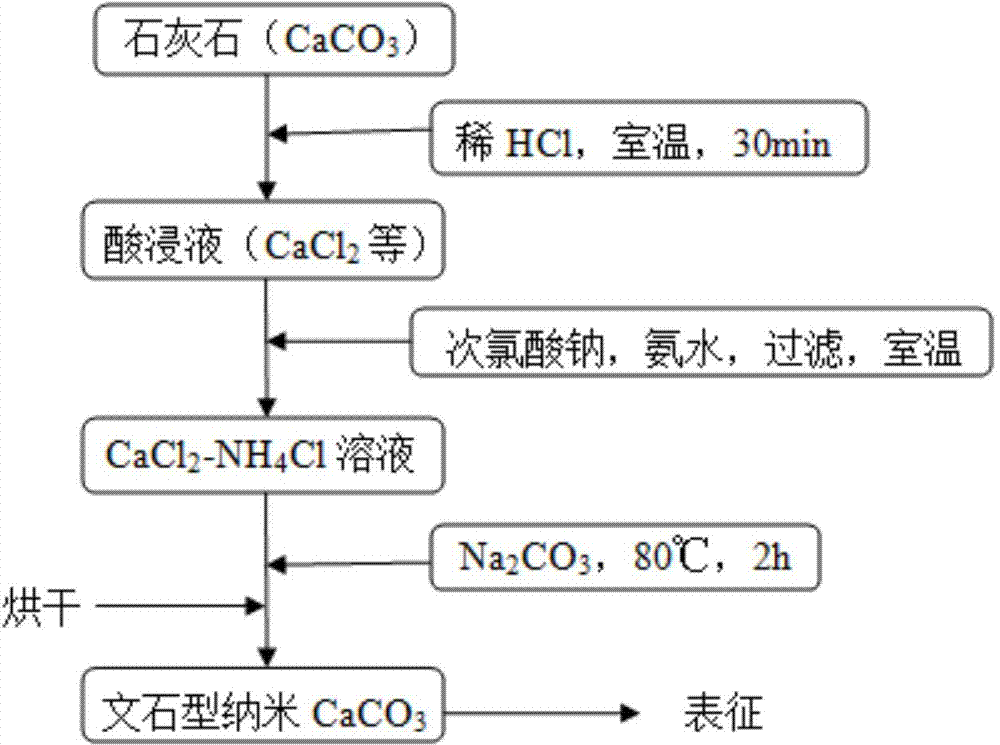
[0029] 1) Grind the limestone ore into a powder about 40μm;
[0030] 2) Add 50g of limestone powder obtained in step 1) to 540mL of 2mol / L hydrochloric acid solution, stir and react at room temperature for 30min, and filter;
[0031] 3) Take 100 mL of the filtrate obtained in step 2) and add 0.04 g of NaClO solution with an available chlorine content of 6 wt%; after the reaction is completed, add 12 mol / L ammonia water, adjust the pH to 6, and obtain iron hydroxide precipitation. After standing for 45 minutes Suction filtration to remove ferric hydroxide; continue to add 12mol / L ammonia water to adjust the pH to 11.00, and let stand for 30 minutes to obtain magnesium hydroxide precipitation, suction filtration to remove magnesium hydroxide to obtain CaCl 2 -NH 4 Cl solution;
[0032] 4) The CaCl obtained in step 3) 2 -NH 4 The Cl solution and the sodium carbonate solution are added into the reaction vessel at the same time in a volume ratio of 1:1. The concentration of the sodium car...
Embodiment 2
[0034] 1) Grind the limestone ore into a powder about 40μm;
[0035] 2) Add 50 g of the limestone powder obtained in step 1) to 720 mL of 1.5 mol / L hydrochloric acid solution, stir and react for 30 min at room temperature, and filter;
[0036] 3) Take 100 mL of the filtrate obtained in step 2) and add 0.05 g of NaClO solution with an effective chlorine content of 6 wt%; after the reaction is completed, add 12 mol / L ammonia water, adjust the pH to 5, and obtain iron hydroxide precipitation. After standing for 45 minutes Suction filtration to remove ferric hydroxide; continue to add 12mol / L ammonia water to adjust the pH to 11.00, and let stand for 30 minutes to obtain magnesium hydroxide precipitation, suction filtration to remove magnesium hydroxide to obtain CaCl 2 -NH 4 Cl solution;
[0037] 4) The CaCl obtained in step 3) 2 -NH 4 The Cl solution is diluted or concentrated to make the concentration of calcium chloride 0.115mol / L, 0.23mol / L, 0.46mol / L, 0.92mol / L, 1.84mol / L, and then...
Embodiment 3
[0039] 1) Grind the limestone ore into a powder about 40μm;
[0040] 2) Add 50g of limestone powder obtained in step 1) to 540mL of 2mol / L hydrochloric acid solution, stir and react at room temperature for 30min, and filter;
[0041] 3) Take 100 mL of the filtrate obtained in step 2) and add 0.04 g of NaClO solution with an available chlorine content of 6 wt%; after the reaction is completed, add 12 mol / L ammonia water and adjust the pH to 5 to obtain iron hydroxide precipitation. After standing for 45 minutes Suction filtration to remove ferric hydroxide; continue to add 12mol / L ammonia water to adjust the pH to 11.00, let stand for 30 minutes to obtain magnesium hydroxide precipitation, suction filtration to remove magnesium hydroxide to obtain CaCl 2 -NH 4 Cl solution;
[0042] 4) The CaCl obtained in step 3) 2 -NH 4 Dilute the Cl solution to a concentration of 0.46mol / L, prepare a sodium carbonate solution of the same concentration and the same molar amount, and then add it to th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com