A kind of synthetic method of n-(2-fmoc-aminoethyl) glycine methyl ester hydrochloride
A technology of glycine methyl ester and synthesis method, which is applied in the field of chemical synthesis of intermediates, can solve problems such as difficult operation, high cost, and low yield, and achieve the effects of reducing usage, simple operation, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
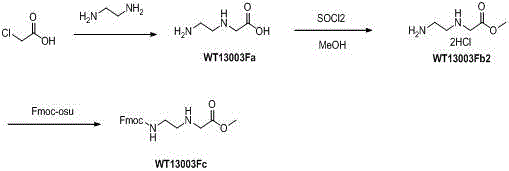
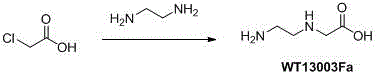
[0014] 1 Synthesis of Wt13003Fa:
[0015]
[0016] At room temperature, chloroacetic acid (800g, 8.47 mol) was added to ethylenediamine (5.5 L ) in batches within three hours, and the stirring was completed overnight, and the excess ethylenediamine was distilled off with an oil pump under reduced pressure to obtain a light yellow The viscous oil was added with DMSO overnight, a white solid precipitated out, filtered, washed twice, and dried to obtain 700 g of crude product (yield: 54.5%, purity 60% as determined by TLC), which was directly used in the next step.
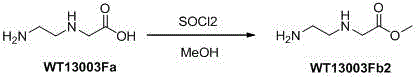
[0017] 2 Synthesis of Wt13003Fb2:
[0018]
[0019] Suspend WT13003Fa (75g, 0.64 mol) in methanol (1.5L), cool to 0°C, add thionyl chloride (230mL, 3.2mol) dropwise, heat and reflux for 4 hours, remove the solvent under reduced pressure with a water pump, and obtain a white product (84g, yield 100%), directly used in the next step.
[0020] 3 Synthesis of Wt13003Fc:
[0021]
[0022] Suspend WT13003Fb2 (7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com