Synthesis method of amorphous calcium carbonate nanoparticles with controllable particle size
A nanoparticle, calcium carbonate technology, applied in the direction of calcium carbonate/strontium/barium, nanotechnology for materials and surface science, nanotechnology, etc., can solve the problem of amorphous calcium carbonate nanoparticles that do not have particle size controllability Problems such as poor particle size uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
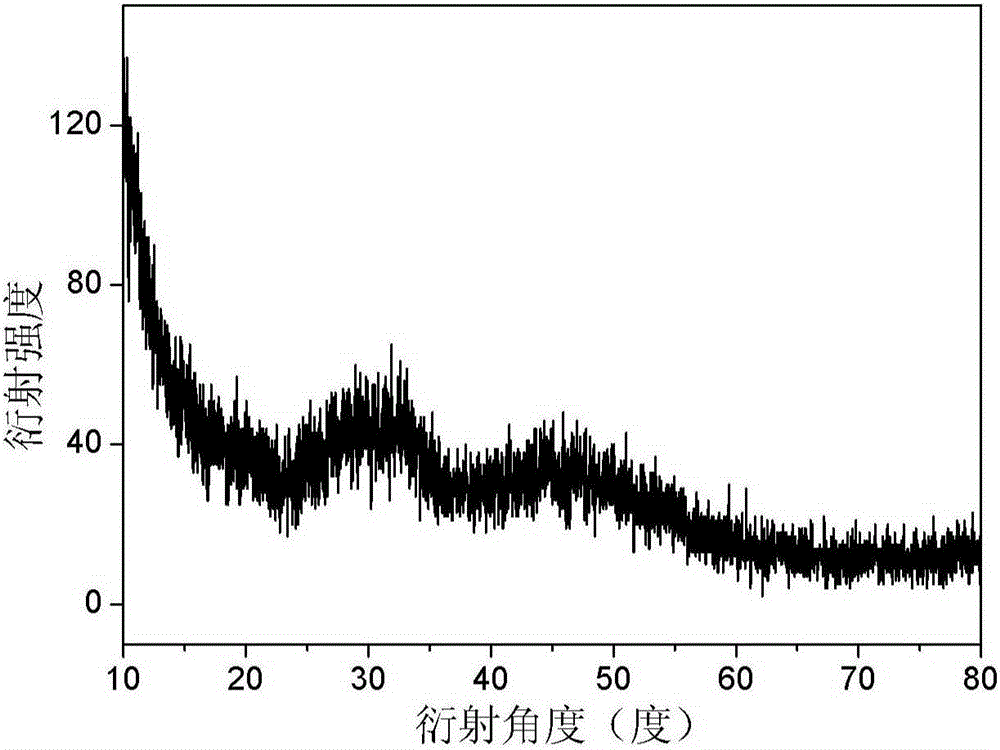
[0041] Add 0.2g of calcium chloride dihydrate to 100ml of absolute ethanol, and add 1ml of water after it is completely dissolved. Place the above liquid in a 150ml glass jar, seal the mouth of the bottle with a parafilm, and reserve a small hole on the parafilm. The ammonium bicarbonate solid was packed into a 20ml glass jar, the mouth of the bottle was sealed with a parafilm, and a small hole was reserved on the parafilm. The above two reactors were placed together in a sealed desiccator, and kept at a constant temperature of 30° C. for 24 hours to obtain a translucent white liquid. The liquid was centrifuged (8,000 rpm, 10 minutes), and the white precipitate was collected and washed with absolute ethanol to obtain amorphous calcium carbonate nanoparticles, which were stored in absolute ethanol. Characterized by X-ray diffraction patterns, such as figure 1 As shown, the characteristic spectrum of amorphous is shown in the spectrum, and there is no characteristic diffractio...
Embodiment 2
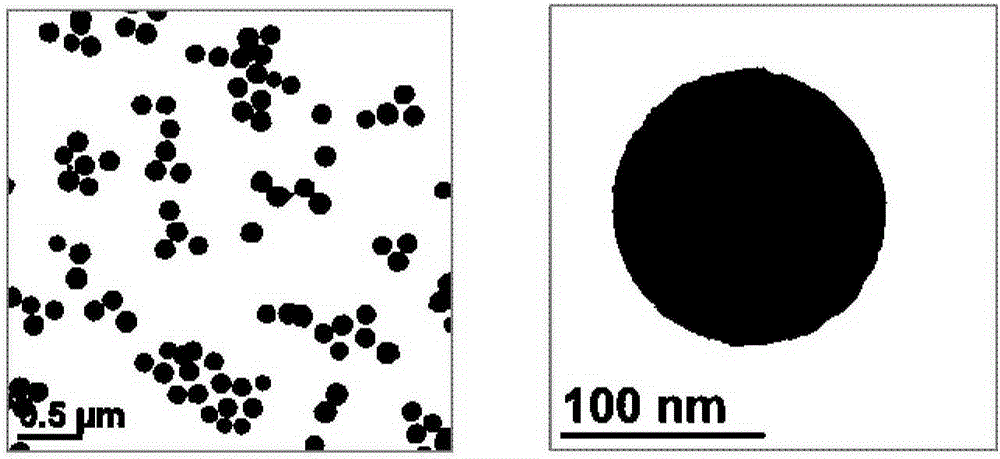
[0043] Add 0.1g of calcium chloride dihydrate to 100ml of absolute ethanol, and add 1ml of water after it is completely dissolved. Place the above liquid in a 150ml glass jar, seal the mouth of the bottle with a parafilm, and reserve a small hole on the parafilm. The ammonium bicarbonate solid was packed into a 20ml glass jar, the mouth of the bottle was sealed with a parafilm, and a small hole was reserved on the parafilm. The above two reactors were placed together in a sealed desiccator, and kept at a constant temperature of 30° C. for 24 hours to obtain a translucent white liquid. The liquid was centrifuged (10,000 rpm, 10 minutes), and the white precipitate was collected and washed with absolute ethanol to obtain amorphous calcium carbonate nanoparticles, which were stored in absolute ethanol. The morphology of such nanoparticles is image 3 As shown, the particle size is 86nm, and the particle size is uniform, forming a monodisperse state.
Embodiment 3
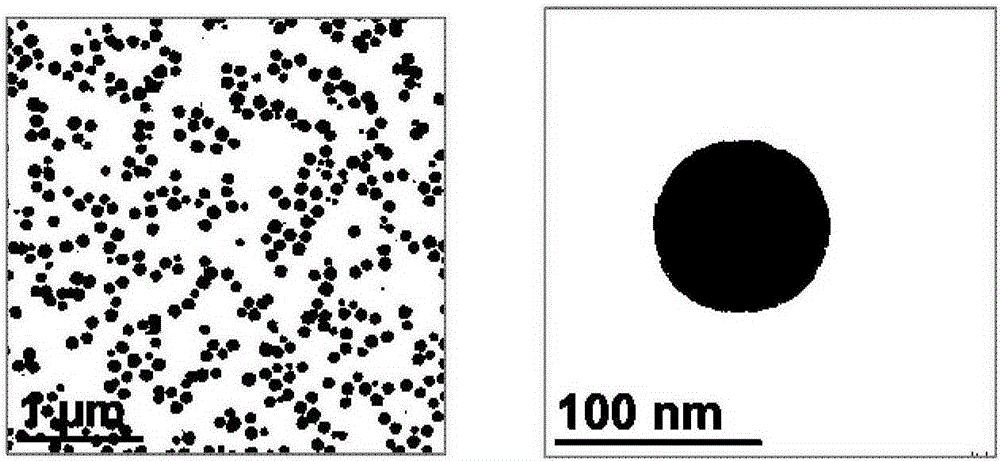
[0045]Add 0.05g of calcium chloride dihydrate into 100ml of absolute ethanol, and add 0.75ml of water after completely dissolving. Place the above liquid in a 150ml glass jar, seal the mouth of the bottle with a parafilm, and reserve a small hole on the parafilm. The ammonium bicarbonate solid was packed into a 20ml glass jar, the mouth of the bottle was sealed with a parafilm, and a small hole was reserved on the parafilm. The above two reactors were placed together in a sealed desiccator, and kept at a constant temperature of 30° C. for 24 hours to obtain a translucent white liquid. The liquid was centrifuged (12,000 rpm, 10 minutes), and the white precipitate was collected and washed with absolute ethanol to obtain amorphous calcium carbonate nanoparticles, which were stored in absolute ethanol. The morphology of such nanoparticles is Figure 4 As shown, the particle size is 56nm, and the particle size is uniform, forming a monodisperse state.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com