A kind of hydroxycinnamic acid amine alkaloid and its preparation and application
An alkaloid and cinnamic acid amine technology, applied in the field of medicinal chemistry and pharmacotherapy, can solve the problems of hypoxia and plant damage, and achieve the effect of inhibiting COMT activity, good physical and chemical properties and oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: the preparation of compound 8,10,19
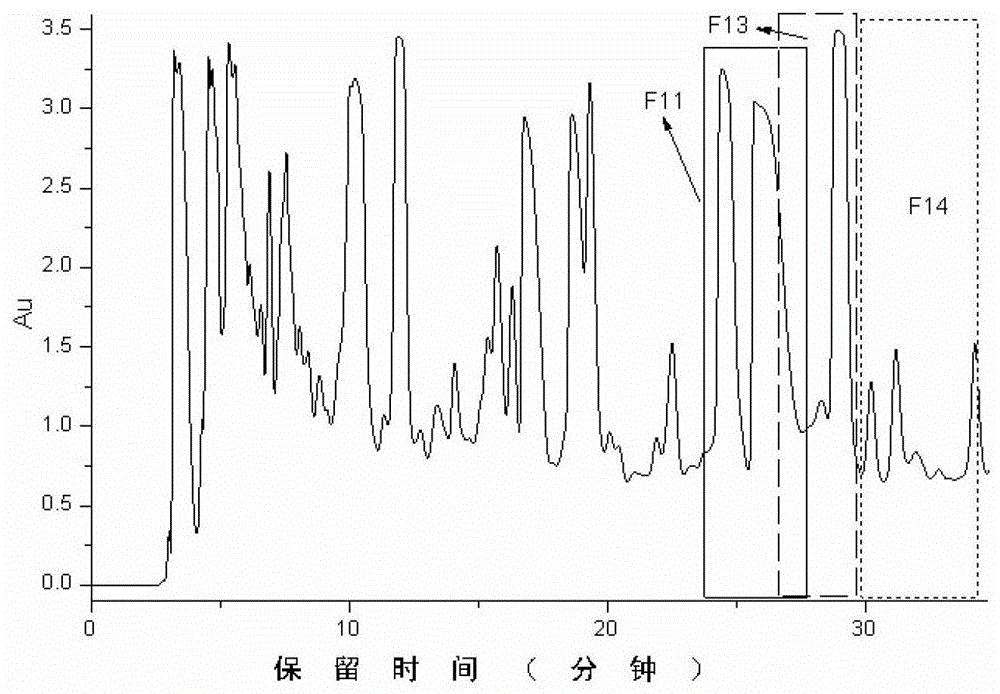
[0054] Compounds 8, 10, and 19 were isolated from Tanggu Tescopolamine, and the pretreatment method was the same as that in patent 201110179917.9. Extract 10 kg of anisodamine with 95% ethanol at 0-100°C, extract 120 minutes each time, extract 2 times in total, obtain a total of 1500 liters of extract, concentrate it at 80°C to 80 liters, and use non- Water SPE method processing. Specifically, take a silica-based strong cation exchange (SCX) SPE column (20 g, 60 ml), activate and equilibrate with 60 ml of pure methanol, and load 75 ml of crude alkali solution. Wash the SPE cartridge with 10-60 mL of methanol to remove the alkaloids in the crude base. Finally, the alkaloids were eluted with 60 ml of 250 mmol per liter of ammonium perchlorate-methanol solution. Finally, we obtained the alkaloid-rich fragment of Anisodora, which contains very little non-alkaloids, which greatly simplifies the quantitative analysis proc...
Embodiment 2
[0063] Embodiment 2: the preparation of compound 9,25
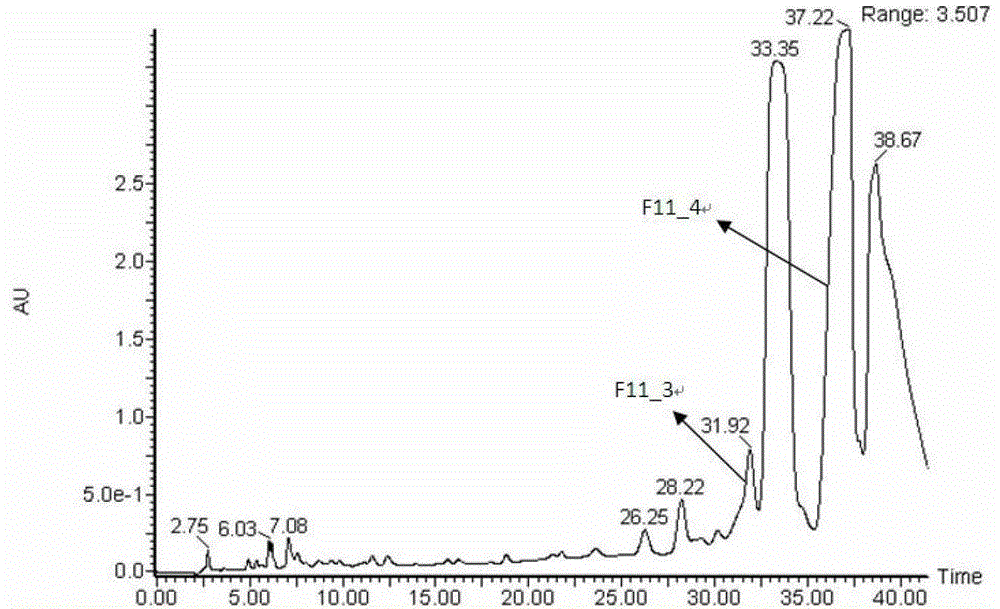
[0064] Compounds 9 and 25 were isolated from Tanggu Tescopolamine, and the pretreatment method was the same as that in patent 201110179917.9. Extract 10 kg of anisodamine with 95% ethanol at 0-100°C, extract 120 minutes each time, extract 2 times in total, obtain a total of 1500 liters of extract, concentrate it at 80°C to 80 liters, and use non- Water SPE method processing. Specifically, take a silica-based strong cation exchange (SCX) SPE column (20 g, 60 ml), activate and equilibrate with 60 ml of pure methanol, and load 75 ml of crude alkali solution. Wash the SPE cartridge with 10-60 mL of methanol to remove the alkaloids in the crude base. Finally, the alkaloids were eluted with 60 ml of 250 mmol per liter of ammonium perchlorate-methanol solution. Finally, we obtained the alkaloid-rich fragment of Anisodora, which contains very little non-alkaloids, which greatly simplifies the quantitative analysis process of a...
Embodiment 3
[0071] Embodiment 3: the preparation of compound 5,20
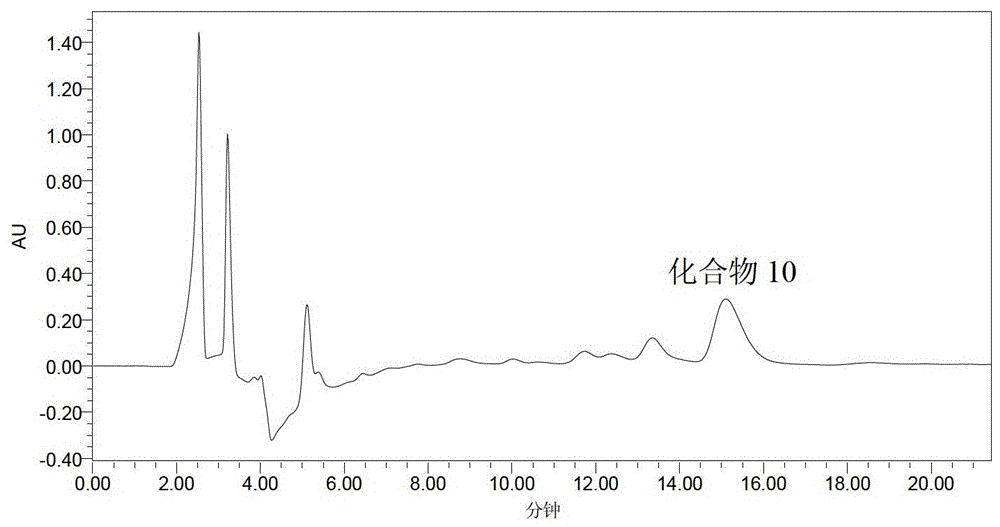
[0072] Compounds 5 and 20 are isolated from Tanggu Tescopolamine, and the pretreatment method is the same as that in patent 201110179917.9. Extract 10 kg of anisodamine with 95% ethanol at 0-100°C, extract 120 minutes each time, extract 2 times in total, obtain a total of 1500 liters of extract, concentrate it at 80°C to 80 liters, and use non- Water SPE method processing. Specifically, take a silica-based strong cation exchange (SCX) SPE column (20 g, 60 ml), activate and equilibrate with 60 ml of pure methanol, and load 75 ml of crude alkali solution. Wash the SPE cartridge with 10-60 mL of methanol to remove the alkaloids in the crude base. Finally, the alkaloids were eluted with 60 ml of 250 mmol per liter of ammonium perchlorate-methanol solution. Finally, we obtained the alkaloid-rich fragment of Anisodamine, which contains very little non-alkaloids, which greatly simplifies the quantitative analysis process of alk...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap