Application of nicotinamide mononucleotide in the preparation of drugs for promoting nerve regeneration after cerebral ischemia
A single nucleotide, nerve regeneration technology, applied in the field of medicine, to achieve the effect of rich variety, wide therapeutic window, and improvement of nerve function damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The brain injury model was prepared by occluding the midvein of the mouse brain. The model preparation process was as follows: the mouse (C57BL / 6J, purchased from Shanghai Sleike Experimental Animal Co., Ltd.) was anesthetized (4% chloral hydrate, 0.1ml / 10g, intraperitoneally After the injection), stroke surgery was performed (①The mouse was selected with 5-0 monofilament nylon suture, and the final diameter of the top was 0.150-0.200 mm. A median incision was made in the ventral neck, and the sternohyoid muscle and sternomastoid were further bluntly separated. The right common carotid artery (CCA) and the right external carotid artery (ECA) were exposed. ②The proximal end of the CCA and the beginning of the external carotid artery were ligated with 3-0 suture. ③The internal carotid artery (ICA) Use a 3-0 suture to loosely ligate, and make sure that no branch is tied.④ 3mm below the common carotid artery bifurcation, use a 6-0 suture to loosely ligate to mark the positio...
Embodiment 2
[0039] The brain injury model was prepared by blocking the midvein of the mouse brain, and the preparation process of the model was the same as in Example 1.
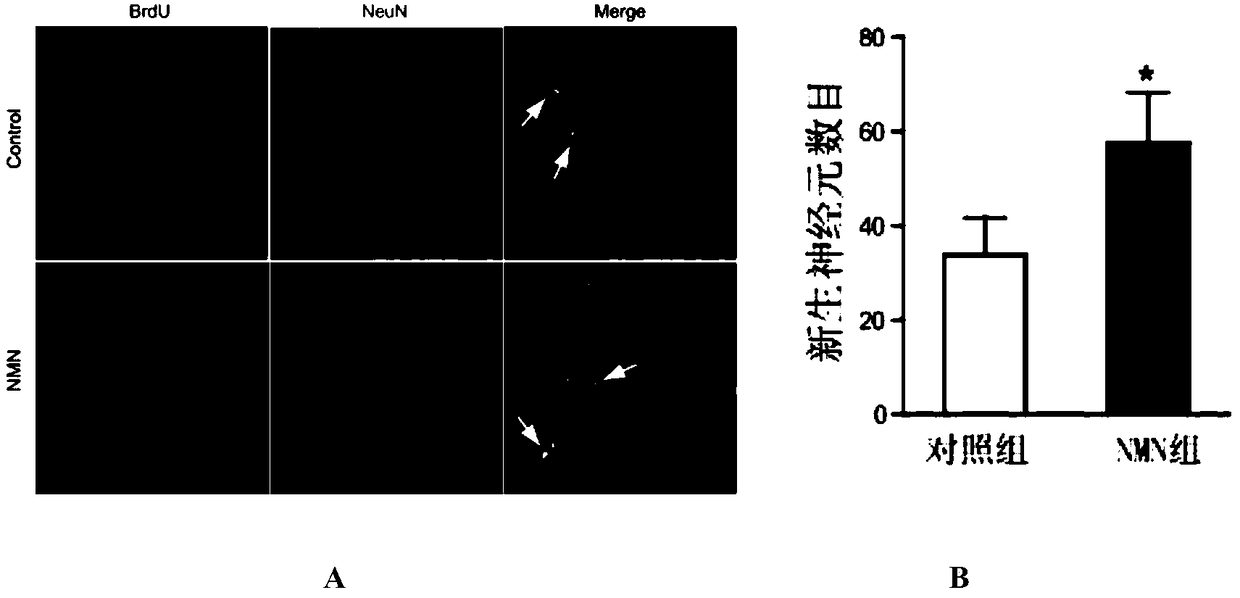
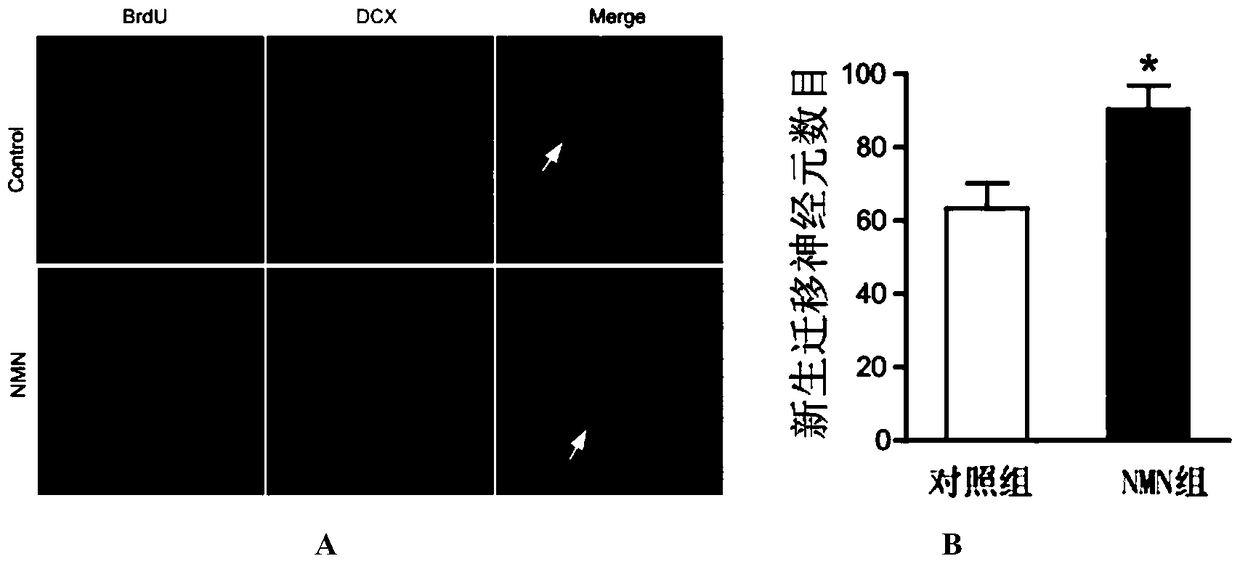
[0040] The administration group was injected intraperitoneally with nicotinamide mononucleotide (NMN) (purchased from Sigma, USA) at 500 mg / kg / day (prepared in normal saline) for 9 consecutive days; the control group was injected with the same amount of normal saline for 9 consecutive days.
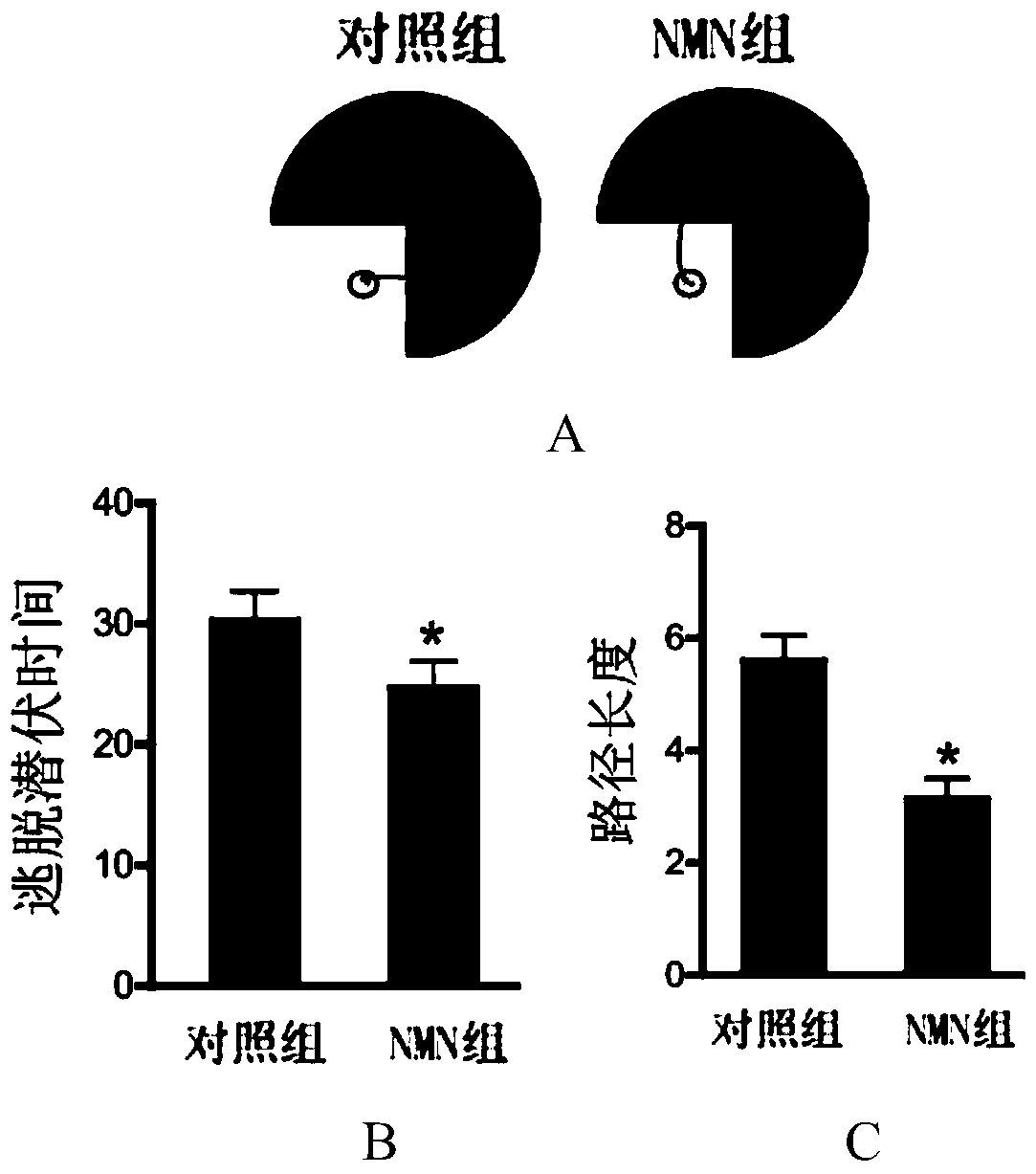
[0041] After 14 days, the Morris water maze test was used to evaluate the learning and memory functions of the two groups of mice. The steps of the Morris water maze experiment are as follows: (1) put the mouse into the water facing the pool wall, and randomly put it into the four directions of east, west, south and north as the starting position. The time (seconds) and the path length (meters) for the mouse to find the underwater platform were recorded. In the first few training sessions, if the mouse finds the underwater platfor...
Embodiment 3
[0044] The neural stem cells cultured in vitro were used, and the control group was given physiological saline and nicotinamide mononucleotide (NMN, purchased from Sigma Company) at a dose of 300 μM.
[0045] The process of culturing neural stem cells in vitro is as follows: Pregnant mice (gestational age 14-15 days) were sacrificed by cervical dislocation. After washing, the fetal mice were taken out and the brain tissue was separated. Use microsurgical scissors to cut up the brain tissue, add 2ml of Accutase digestive enzyme dropwise, put it into the cell culture incubator, and digest it for 15-20 minutes. After digestion, an equal volume of neural stem cell culture medium was added to terminate the digestion. After filtering through a 200-mesh nylon mesh, centrifuge at 1000 rpm for 10 minutes, discard the supernatant, and collect the cells. After the cells were resuspended, they were inoculated in neural stem cell medium. Put them in a 37°C, 5% carbon dioxide incubator an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com