Oligosaccharide compound inhibiting endogenous factor X enzyme activity and pharmaceutical composition thereof
A technology of compounds and sugars, applied in the field of invention in the field of medical technology, can solve the problems of complex chemical structure of FG, no research reports on FG compounds, and lack of in-depth research and full understanding of the basic chemical structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
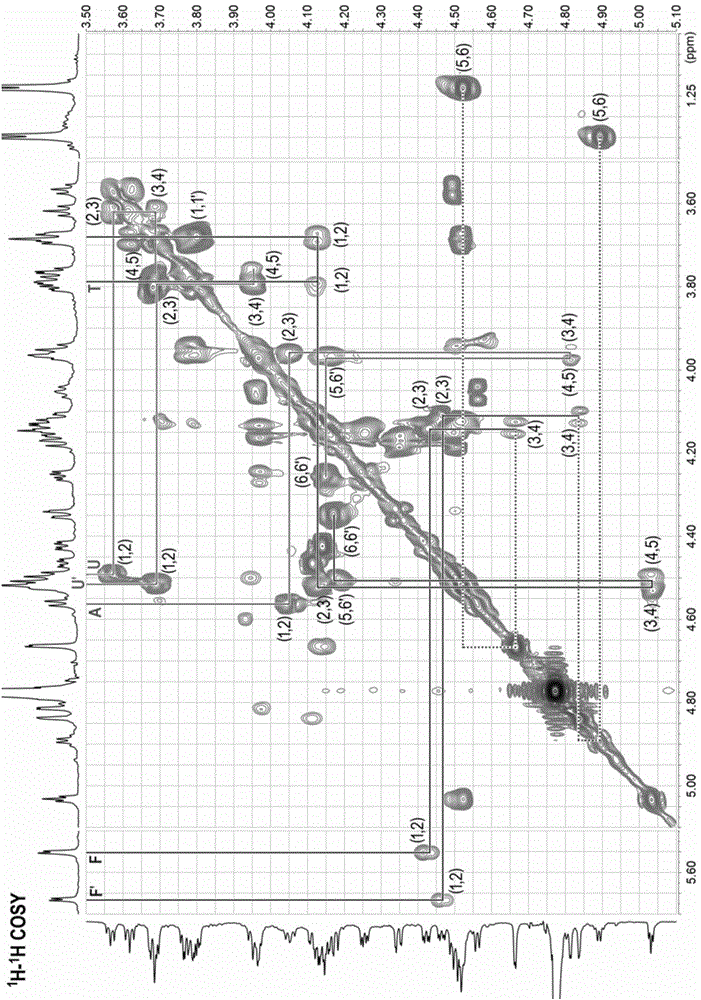
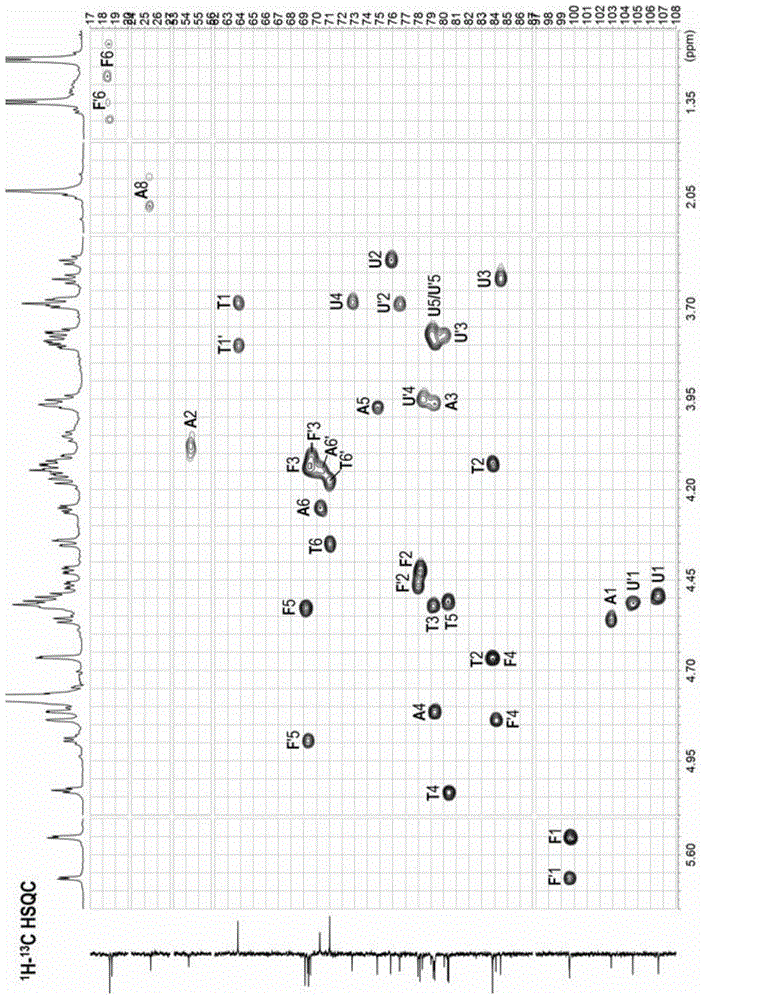
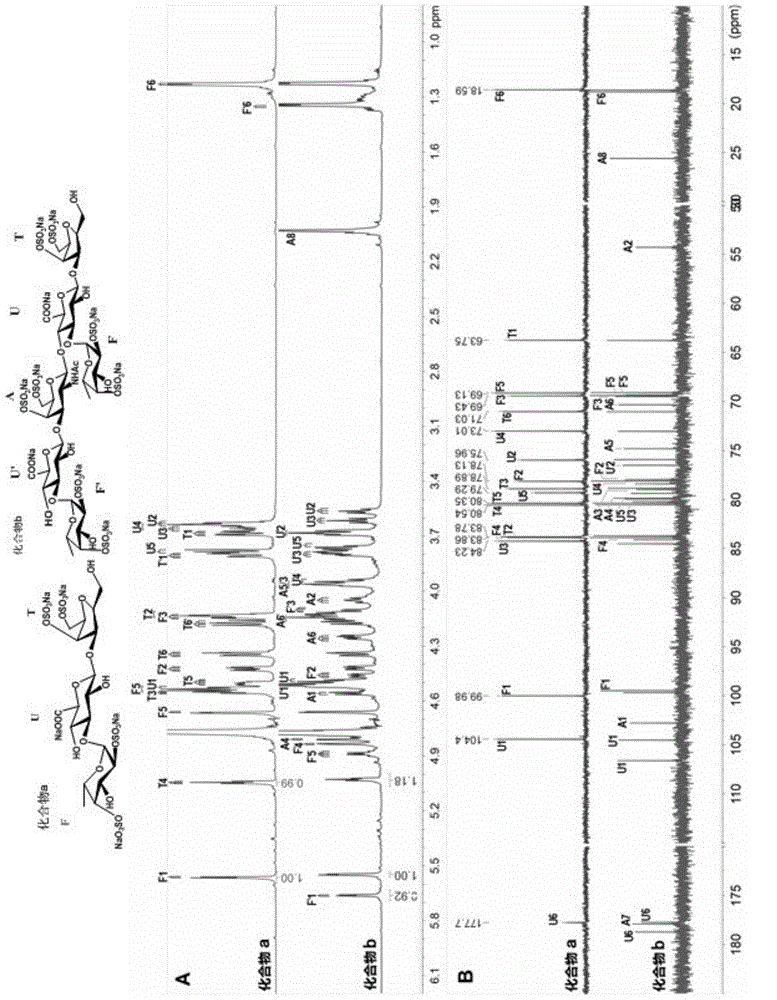
[0074] Preparation of compound a and compound b: L-2,4-disulfated fucosyl-(α1,3)-D-glucuronyl-(β1,3)-2,5-anhydrotalitol (trisaccharide); L-2,4-disulfated fucosyl-(α1,3)-D-glucuronyl-(β1,3)-D-N-acetyl-2-deoxy-2-amino -4,6-disulfated galactosyl-(β1,4)-[L-2,4-disulfated fucosyl-(α1,3)-]D-glucuronyl-(β1, 3) -2,5-anhydrotalitol (hexasaccharide)
[0075] 1.1 Materials
[0076] Fucosylated glycosaminoglycan (FG, sodium salt) derived from the body wall of Stichopus Variegatus Semper was extracted and purified according to the literature method (Marine Drugs, 2013, 11, 399–417), with a weight average molecular weight of about 70kDa. Hydrazine hydrate, hydrazine sulfate, sodium chloride, sodium nitrite, concentrated sulfuric acid, sodium borohydride, absolute ethanol, sodium hydroxide and other reagents are commercially available analytical reagents.
[0077] 1.2 Method
[0078]Step 1. Preparation of partially deacetylated FG: Weigh 2.0 g of FG raw material from Stichopus Variegatus...
Embodiment 2
[0097] Preparation of compounds c~f: L-2,4-disulfated fucosyl-(α1,3)-D-glucuronyl-(β1,3)-{D-N-acetyl-2-deoxy- 2-amino-4,6-disulfated galactosyl-(β1,4)-[L-2,4-disulfated fucosyl-(α1,3)-]D-glucuronyl- (β1,3)-} n -2,5-Anhydrotalitol. In compound c, n=2; in compound d, n=3; in compound e, n=4; in compound f, n=5.
[0098] 2.1 Materials
[0099] With embodiment 1.
[0100] 2.2 Method
[0101] Step 1. Preparation of partially deacetylated FG: Weigh 2.0 g of FG (sodium salt) derived from Stichopus Variegatus, and treat it with the deacetylation reaction and post-treatment method described in Example 1, but the hydrazinolysis reaction time is about 18 hours. About 1.93 g of the deacetylated intermediate product sample was obtained through the reaction, and the yield was about 96.5%. 1 The deacetylation rate of the obtained product detected by H NMR was 40%.
[0102] Step 2. Deamination and depolymerization product preparation: Take 1.50 g of the partially deacetylated intermedi...
Embodiment 3
[0122] Preparation of compounds g~m: L-2,4-disulfated fucosyl-(α1,3)-D-glucuronyl-(β1,3)-{D-N-acetyl-2-deoxy- 2-amino-4,6-disulfated galactosyl-(β1,4)-[L-2,4-disulfated fucosyl-(α1,3)-]D-glucuronyl- (β1,3)-} n -2,5-anhydrotalose diol. In compound g, n=0; in compound h, n=1; in compound i, n=2; in compound j, n=3; in compound k, n=4; in compound l, n=5, compound In m, n=6.
[0123] 3.1 Materials
[0124] The fucosylated glycosaminoglycan (FG, sodium salt) derived from the body wall of Stichopus monotuberculatus was extracted and purified according to the literature method (Marine Drugs, 2013, 11, 399–417), with a weight average molecular weight of about 68kDa. Other reagents and materials are the same as in Example 1.
[0125] 3.2 Method
[0126] Step 1. Preparation of partially deacetylated FG: Take 2.0 g of FG raw material from Stichopus monotuberculatus, and use the same deacetylation reaction and post-treatment method as described in Example 1 with slight changes. Wh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap