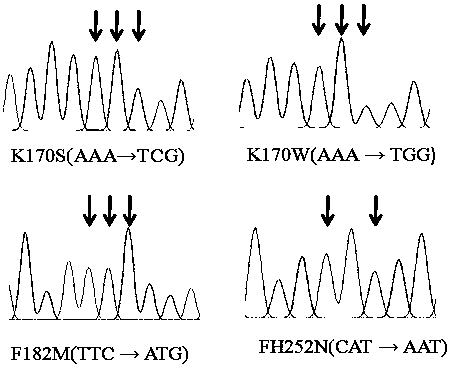
A kind of c-type β-glucosidase mutant and its expression plasmid and recombinant bacteria
A technology of glucosidase and mutants, which is applied in the field of C-type β-glucosidase mutants and their expression plasmids and recombinant bacteria, which can solve the problems of difficult enzyme purification and low catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Construction of mutant expression plasmids and acquisition of recombinant Escherichia coli
[0029] 1. Construction of mutant expression plasmids
[0030] According to protein structure prediction and homologous sequence analysis, a series of mutation sites were found, and point mutations were carried out on these amino acid sites to obtain a series of mutant enzymes. After protein expression and verification, it was found that the catalytic activity of several mutant enzymes was significantly enhanced. The build process is as follows:
[0031] (1) RNA was extracted from the salivary glands and intestinal tissues of domestic termites reared in the laboratory, and cDNA was obtained after reverse transcription.
[0032] (2) The β-glucosidase gene ( JN565079 ), according to the sequence homology, design amplification primers, amplify the termite cDNA obtained in step (1), after PCR amplification, insert the amplified product into the corresponding polyclonal...
Embodiment 2
[0054] Example 2: Activity determination of C-type β-glucosidase before and after mutation
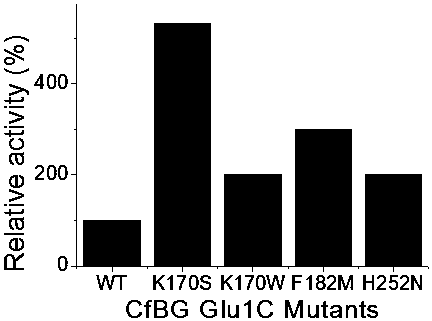
[0055] According to the method in Example 1, the original Escherichia coli pET-28a-Glu1C and other mutant strains were induced to express, and the enzyme activity was measured, and the results were as follows image 3 shown, by attaching image 3 It can be seen that the enzyme activity of the strain after the mutation was increased by 5.2 times, 2.05 times, 2.9 times, and 2.1 times, respectively, compared with that before the mutation.
[0056] The activity of the enzyme after the mutation described in the present invention is greatly improved compared with that before the mutation. The results show that mutating amino acids at key sites can change the activity and other properties of the enzyme. This strategy can be widely used in the improvement of enzymatic properties and accelerates the application of enzymes in industrial production, which has important social significance.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com