Pharmaceutical composition for treating traumatic craniocerebral trauma and preparation method of pharmaceutical composition
A craniocerebral trauma and composition technology, which is applied in the field of pharmaceutical compositions for the treatment of traumatic craniocerebral trauma and its preparation, can solve the problems of lack of original basic research and achieve the effect of alleviating neuroinflammation and alleviating behavioral abnormalities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
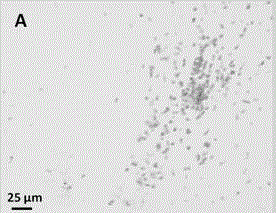
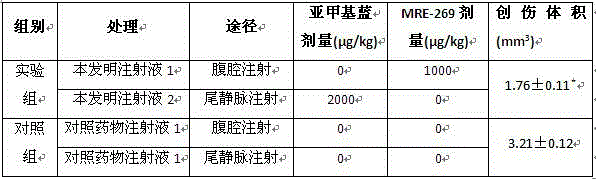
Image
Examples
Embodiment 1
[0023] A pharmaceutical preparation for treating traumatic craniocerebral trauma, prepared by the following method:
[0024] Prescription: 3,7-bis(dimethylamino)phenothiazine-5-onium chloride 40mg, [4-[(5,6-diphenylpyrazinyl)(1-methylethyl)amino] Butoxy]acetic acid 13 mg.
[0025] The pharmaceutical preparation is an injection preparation, and its preparation process is: take the above-mentioned 3,7-bis(dimethylamino)phenothiazine-5-onium chloride and [4-[(5,6-diphenylpyrazinyl ) (1-methylethyl) amino] butoxy] acetic acid, add physiological saline for injection to 100ml, potting and sterilizing to obtain the injection preparation.
[0026] Usage and dosage: Intravenous injection, 10-50ml at a time, once at 0.5h after craniocerebral injury.
Embodiment 2
[0028] A pharmaceutical preparation for treating traumatic craniocerebral trauma, prepared by the following method:
[0029] Prescription: 3,7-bis(dimethylamino)phenothiazine-5-onium chloride 8mg, [4-[(5,6-diphenylpyrazinyl)(1-methylethyl)amino] Butoxy]acetic acid 2.6 mg.
[0030] The pharmaceutical preparation is an injection preparation, and its preparation process is: take the above-mentioned 3,7-bis(dimethylamino)phenothiazine-5-onium chloride and [4-[(5,6-diphenylpyrazinyl ) (1-methylethyl)amino]butoxy]acetic acid, add physiological saline for injection to 1L, fill and sterilize to obtain the injection preparation.
[0031] Usage and dosage: intravenous drip, 500-1000ml once, used 0.5h after craniocerebral injury.
Embodiment 3
[0033] A pharmaceutical preparation for treating traumatic craniocerebral trauma, prepared by the following method:
[0034] Prescription: 3,7-bis(dimethylamino)phenothiazin-5-onium chloride 76.6g, [4-[(5,6-diphenylpyrazinyl)(1-methylethyl)amino ]butoxy]acetic acid 25.5g.
[0035] The preparation process of the injection is: take the above-mentioned 3,7-bis(dimethylamino)phenothiazine-5-onium chloride and [4-[(5,6-diphenylpyrazinyl)(1-methyl Base ethyl) amino] butoxy] acetic acid, 150g of mannitol are mixed, add physiological saline for injection to 1000ml, adjust the pH value of the medicinal solution to 7, filter, pot, and freeze-dry to obtain the freeze-dried powder for injection.
[0036] Usage and dosage: intravenous injection or intravenous infusion, 50-300mg once, used 0.5h after craniocerebral injury.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com