Synthesis method of pyraclostrobin
A technology of pyraclostrobin and a synthetic method, which is applied in the field of synthesis of pyraclostrobin, can solve the problems of few by-products, difficult separation, difficult purification of final products, etc., and achieve the effect of low production cost
Inactive Publication Date: 2015-05-06
ANHUI COSTAR BIOCHEM CO LTD
View PDF4 Cites 39 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
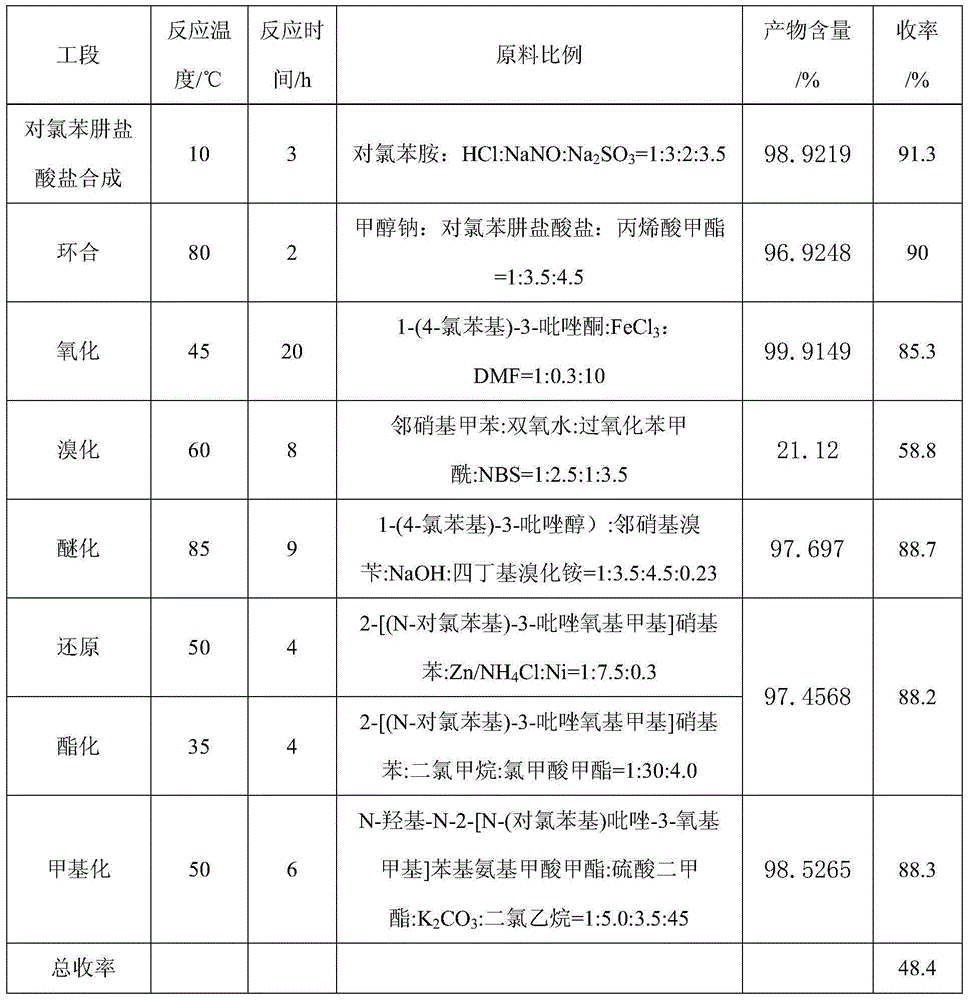
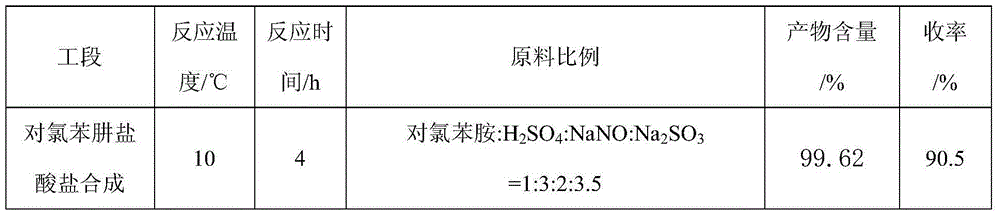
The first condensation method is to prepare pyraclostrobin through the eight-step reaction of p-chlorophenylhydrazine hydrochloride synthesis, cyclization, oxidation, bromination, etherification, reduction, esterification and methylation. The yield is high, and the conditions are relatively mild; the post-condensation method is to prepare pyraclofenac by synthesis, cyclization, oxidation, reduction, esterification, methylation, bromination and condensation reactions of p-chlorophenylhydrazine hydrochloride ester, but the yield of the reduction of o-nitrotoluene to hydroxylamine in this method is low, the condensation yield in the last step is low, and the final product is difficult to purify
At present, the key problem in the synthesis route of pyraclostrobin is the key steps of reduction and bromination reactions, the reaction is easy to produce by-products, and the separation is quite difficult
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0029]
Embodiment 2
[0031]
[0032]
Embodiment 3
[0034]
[0035]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention aims at providing a synthesis method of pyraclostrobin. The method comprises the following steps: with chloroaniline and ortho-nitrotoluene as raw materials, synthesizing, cyclizing, oxidizing, bromizing, etherifying, reducing, esterifying and methylating chlorophenylhydrazine hydrochloride to prepare the pyraclostrobin. The purity of the pyraclostrobin synthesized through the method disclosed by the invention is 98.0% and more, the yield reaches 48%. And the process is low in cost, simple to operate and easy for industrial production.
Description
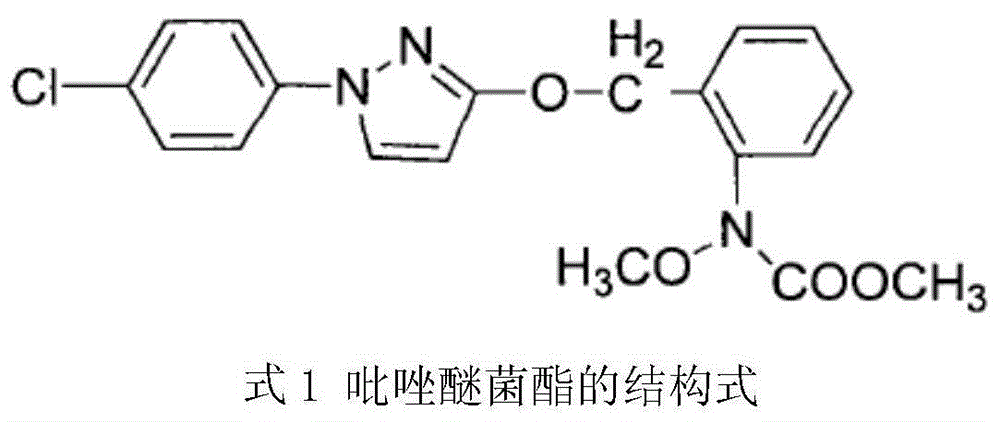
technical field [0001] The invention relates to the field of pesticide fungicides, in particular to a method for synthesizing pyraclostrobin. Background technique [0002] Pyraclostrobin, also known as pyraclostrobin, is a broad-spectrum fungicide of methyl methoxyacrylate with a pyrazole structure discovered by BASF in 1993, with molecular formula C 19 h 18 ClN 3 o 4 , whose structure is [0003] [0004] Pyraclostrobin has patent rights in the United States, Europe and other countries, and has also applied for a patent in my country, but its synthesis technology has not yet been disclosed. At present, the synthetic route of pyraclostrobin at home and abroad can be divided into the following two types: the first condensation method and the post-condensation method, both of which are based on o-nitrotoluene and p-chloroaniline as starting materials. The first condensation method is to prepare pyraclostrobin through the eight-step reaction of p-chlorophenylhydrazine h...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D231/22
CPCC07D231/22
Inventor 谷顺明刘敏王红伟王晓亭
Owner ANHUI COSTAR BIOCHEM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com