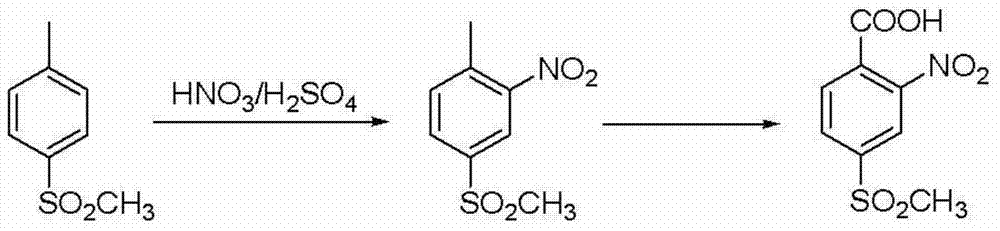
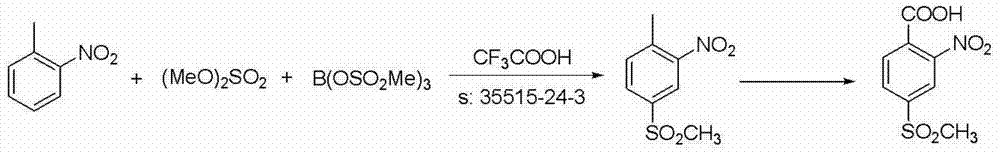
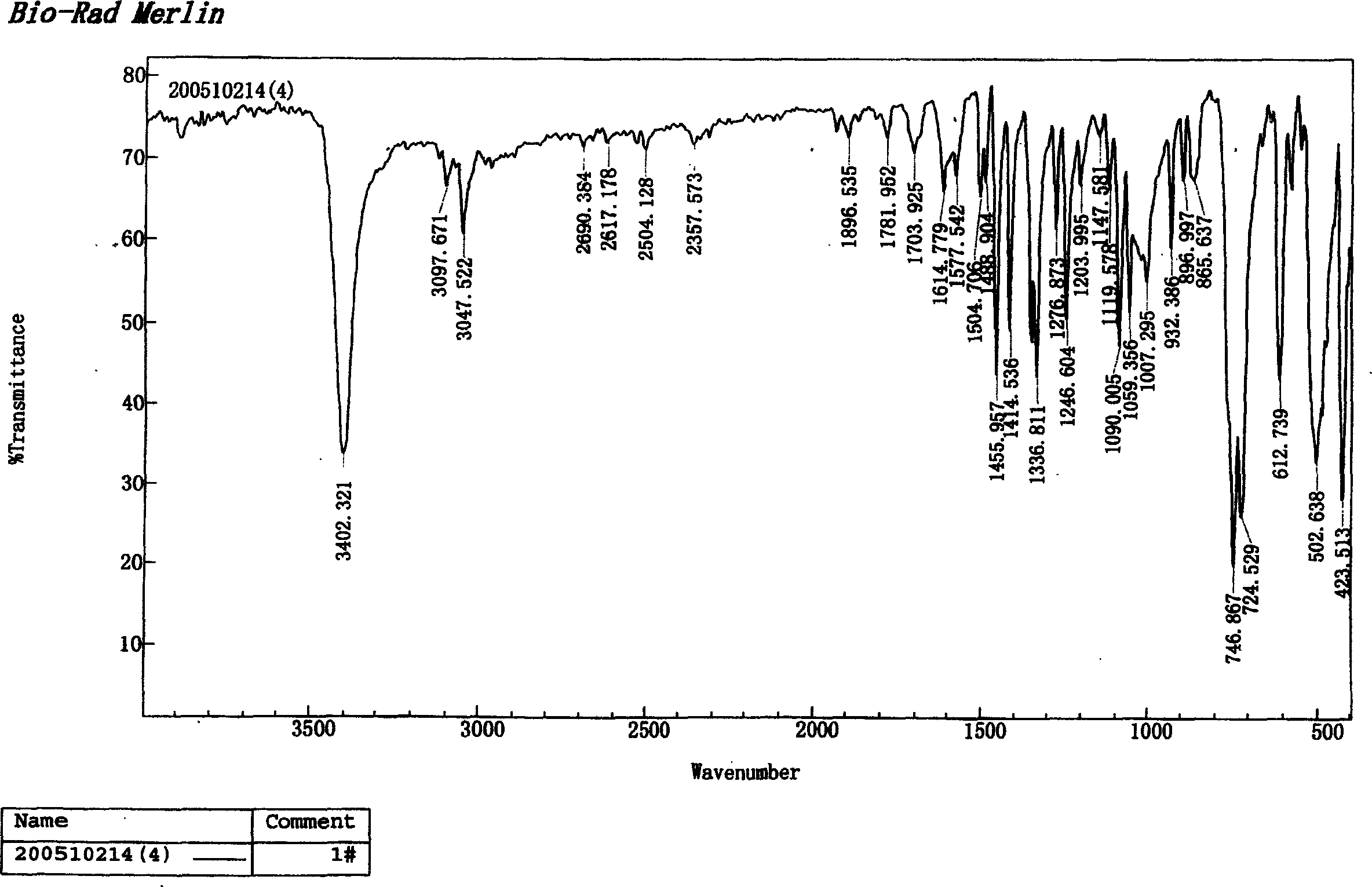
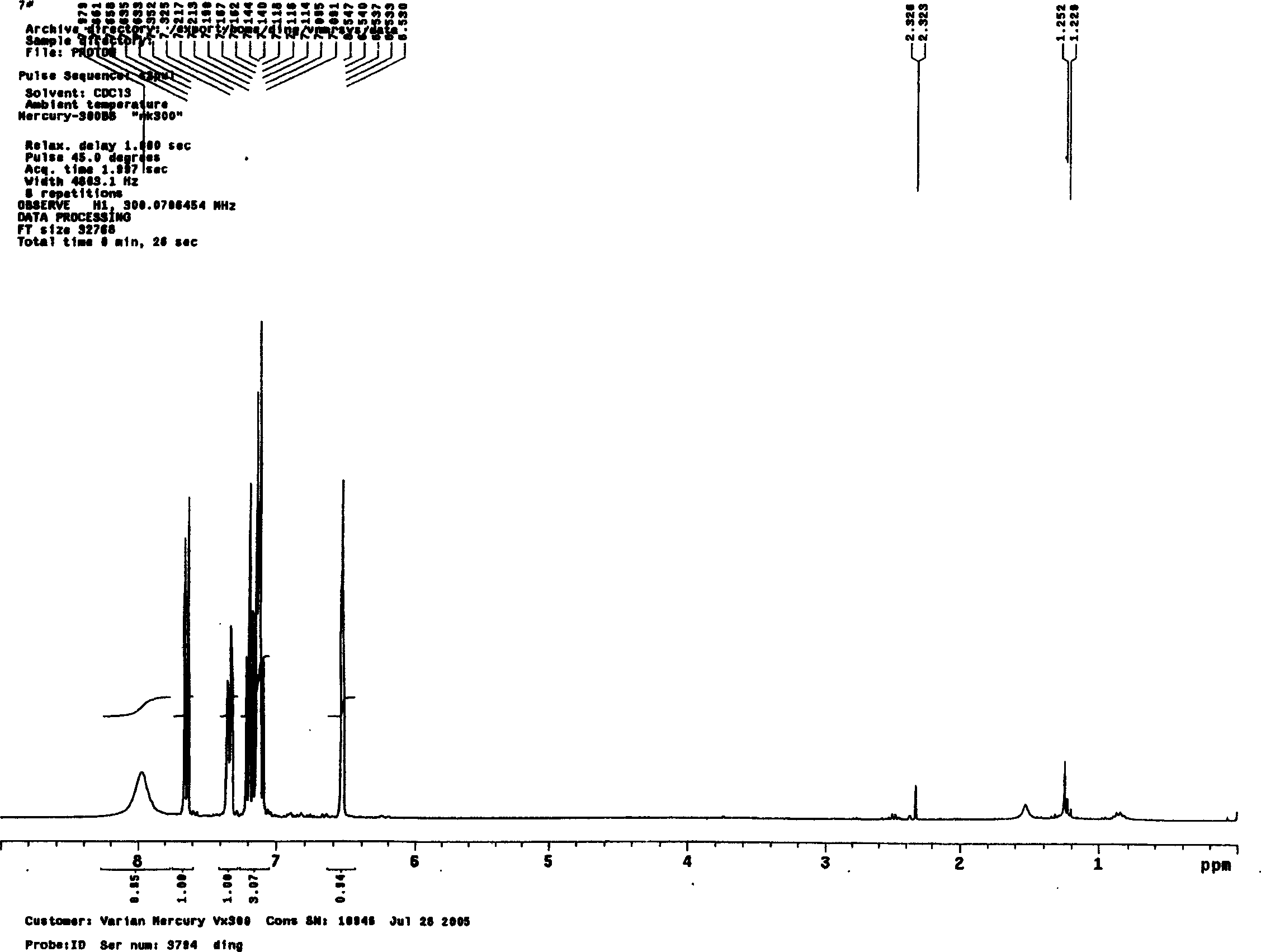
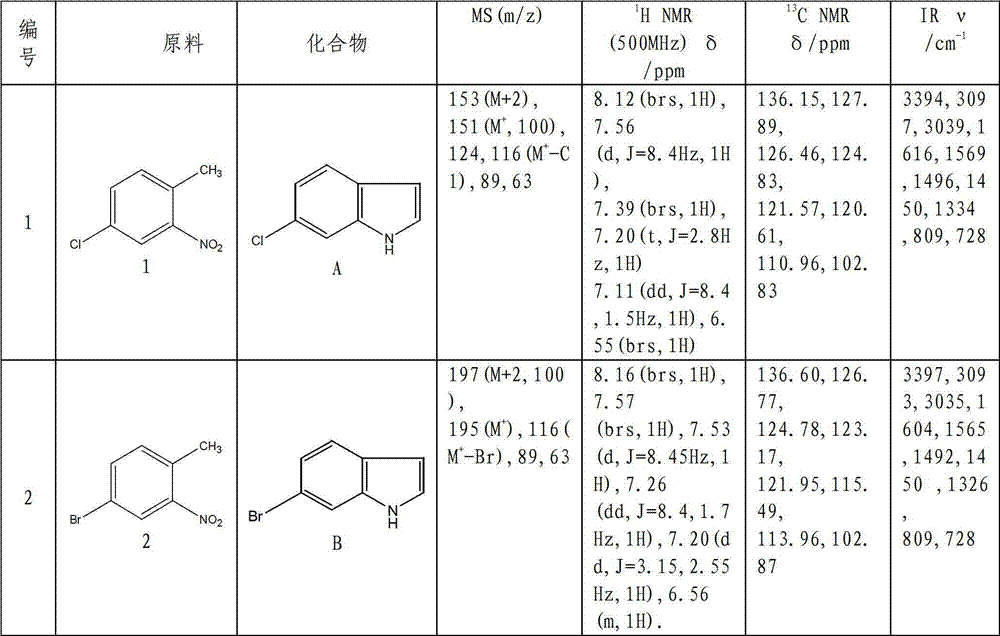
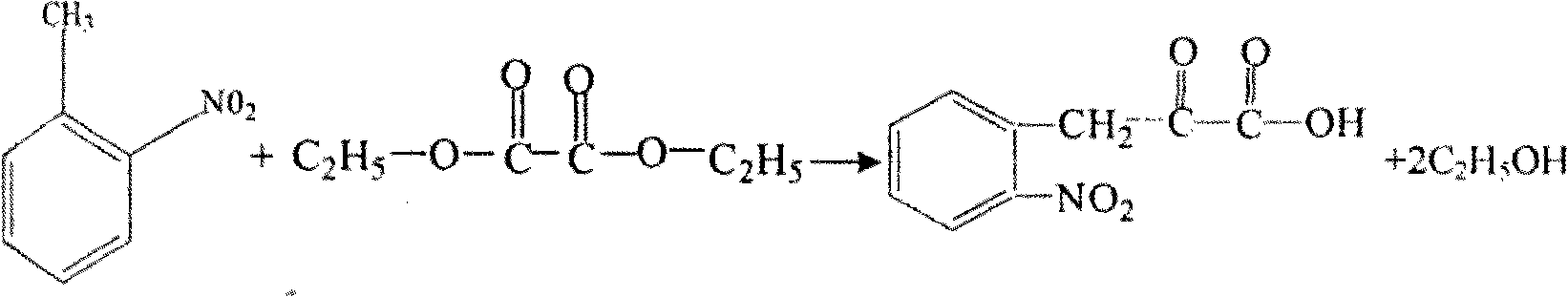
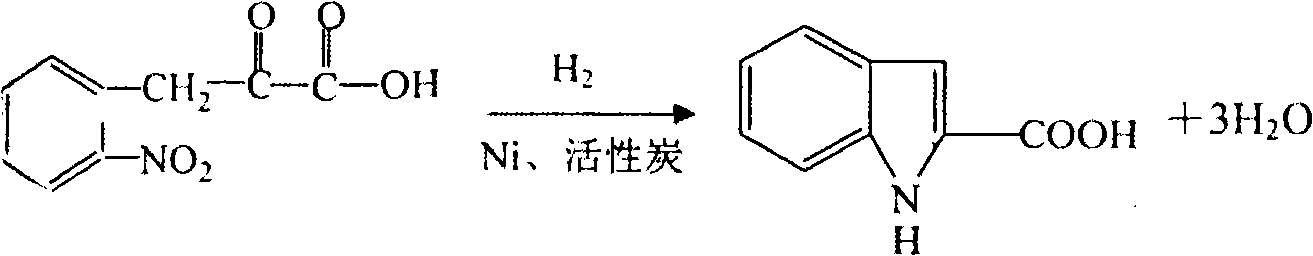
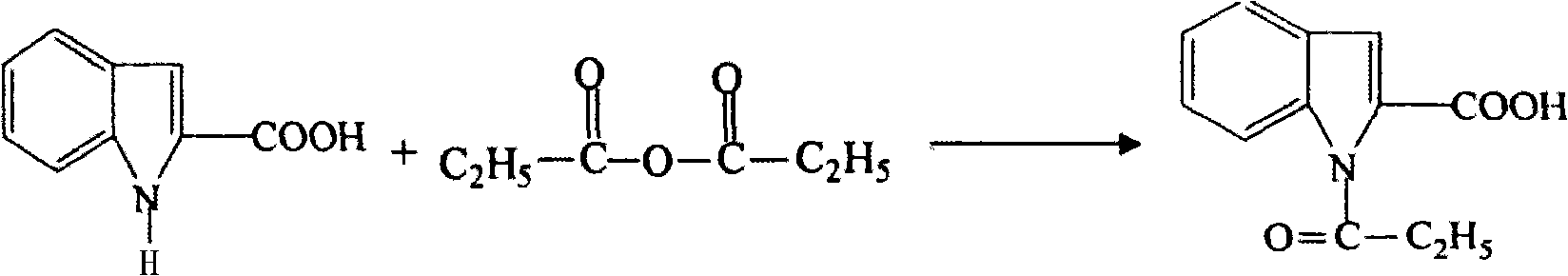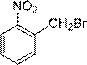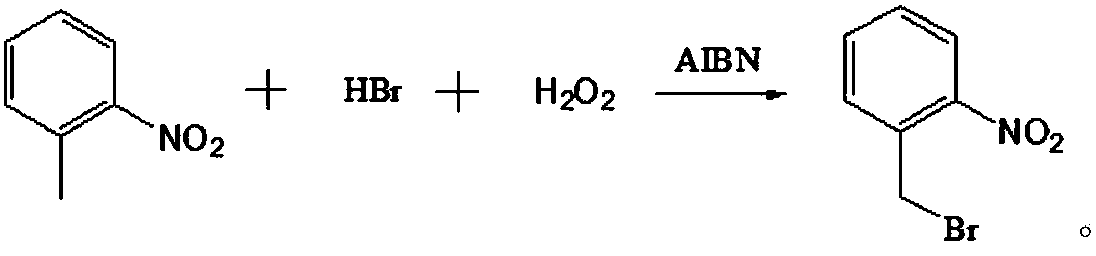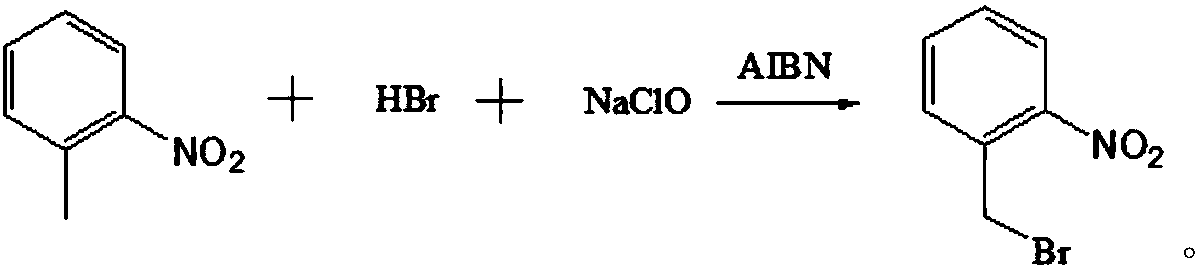Patents
Literature
49 results about "Ortho-nitrotoluene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
2-Nitrotoluene or ortho-nitrotoluene is an organic compound with the formula CH 3 C 6 H 4 NO 2. It is pale yellow liquid that is a versatile intermediate in the production of various dyes. It is pale yellow liquid that is a versatile intermediate in the production of various dyes.
Synthesis method of pyraclostrobin
InactiveCN104592117AReduce generationReduce manufacturing costOrganic chemistrySynthesis methodsHydrochloride
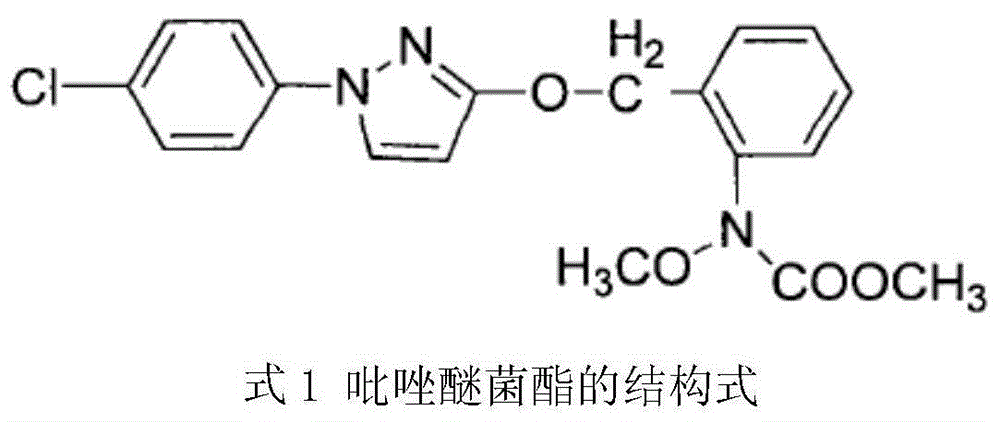
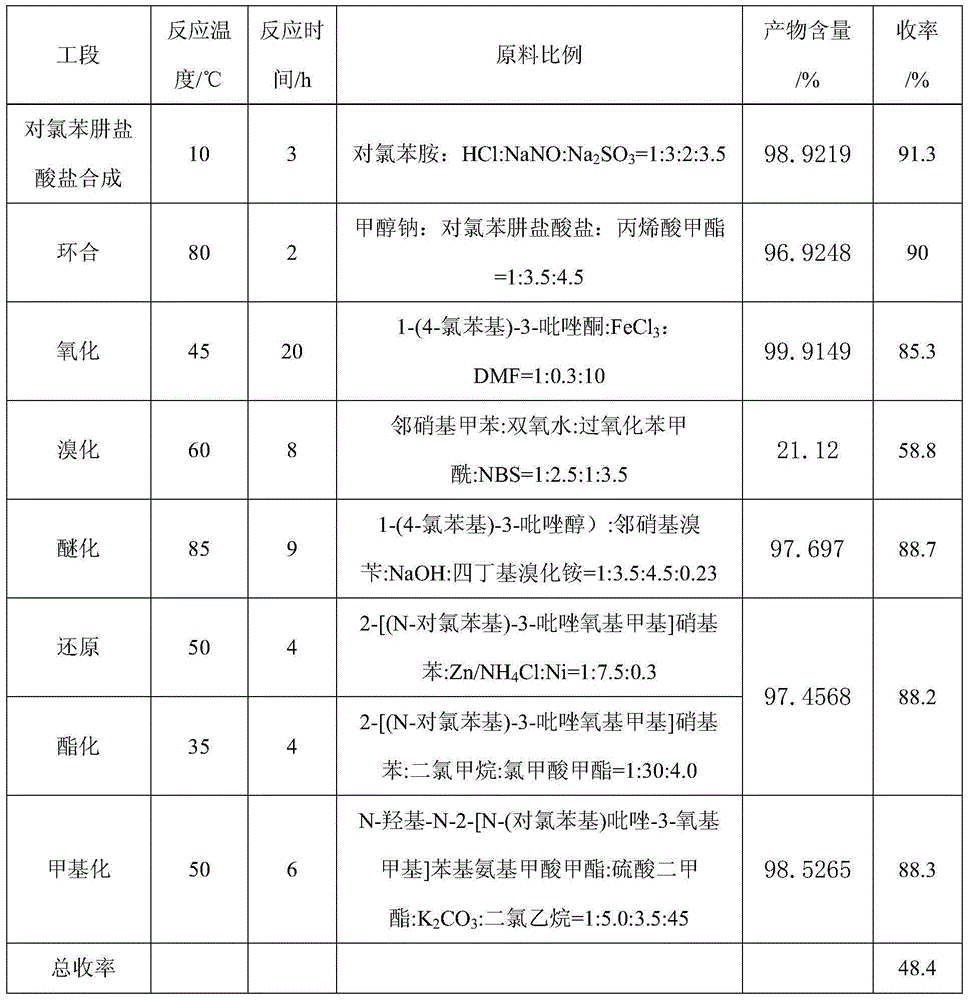
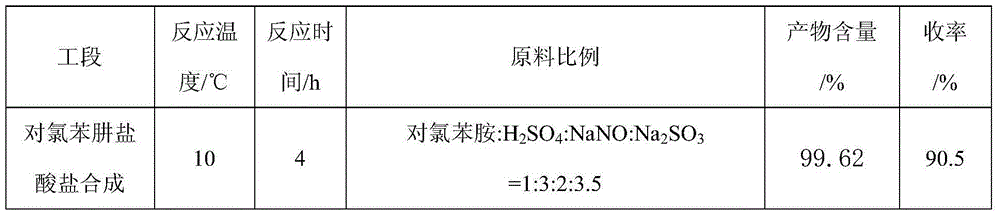
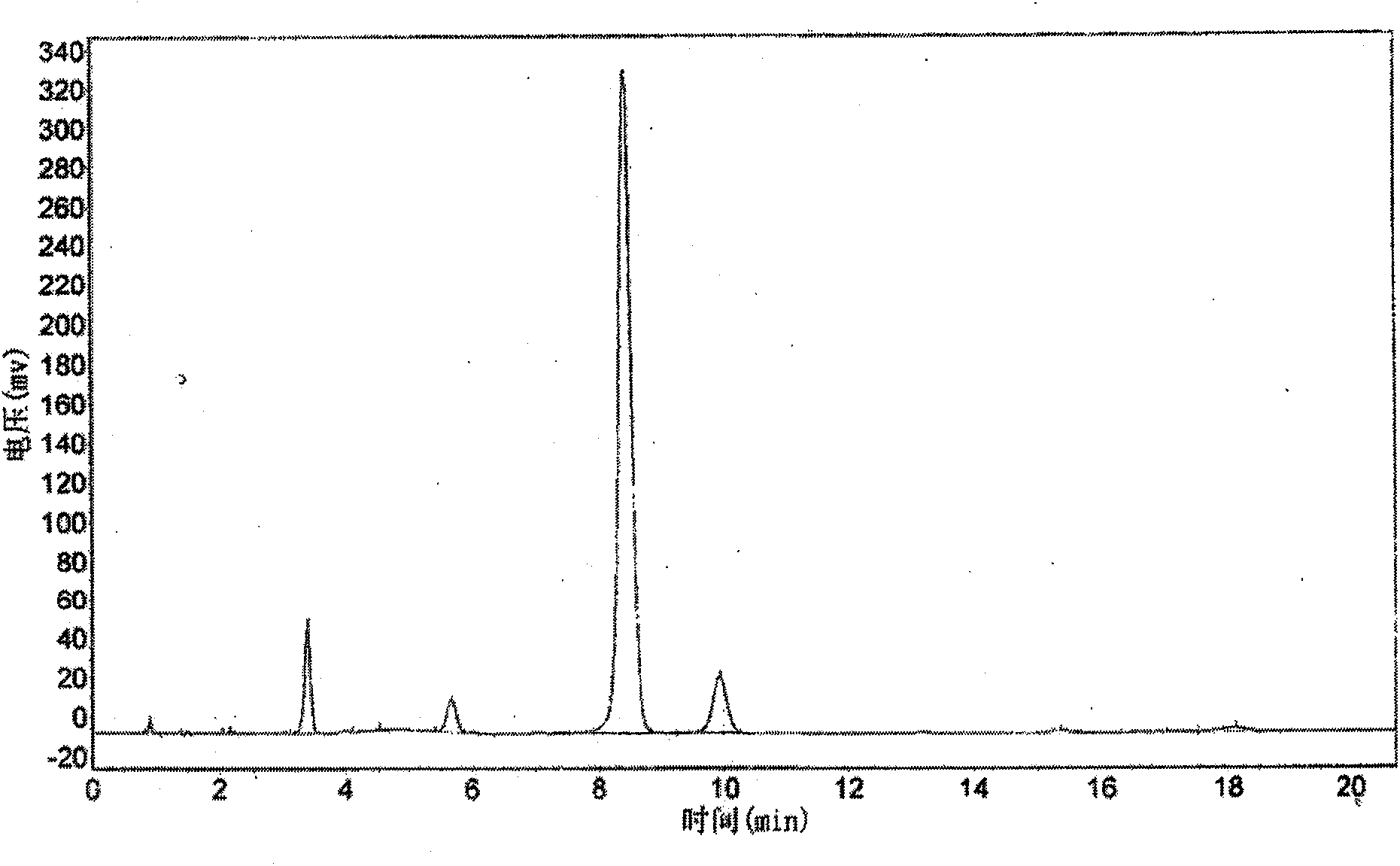
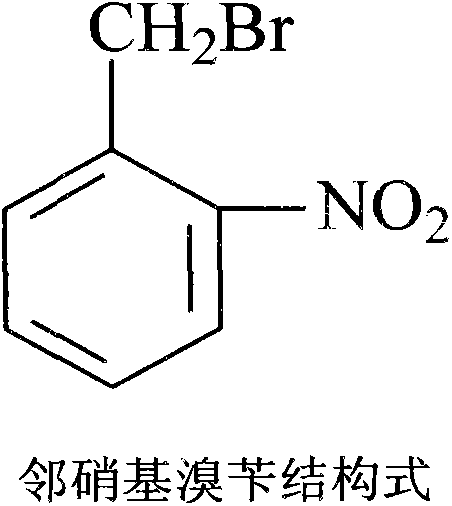
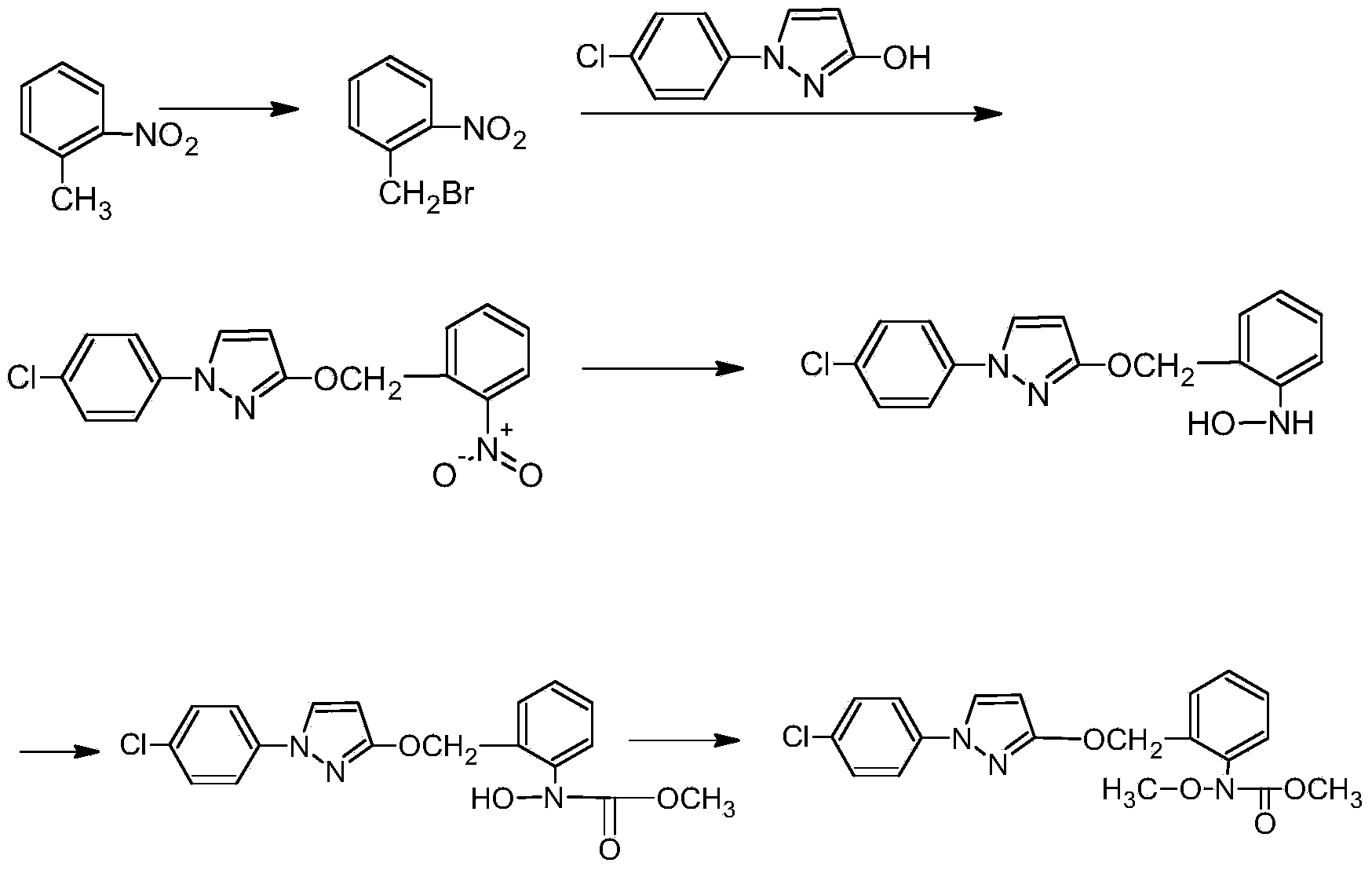
The invention aims at providing a synthesis method of pyraclostrobin. The method comprises the following steps: with chloroaniline and ortho-nitrotoluene as raw materials, synthesizing, cyclizing, oxidizing, bromizing, etherifying, reducing, esterifying and methylating chlorophenylhydrazine hydrochloride to prepare the pyraclostrobin. The purity of the pyraclostrobin synthesized through the method disclosed by the invention is 98.0% and more, the yield reaches 48%. And the process is low in cost, simple to operate and easy for industrial production.
Owner:ANHUI COSTAR BIOCHEM CO LTD
Preparation method of mesotrione
ActiveCN103772243AHigh puritySmooth realization of assembly line productionOrganic chemistryOrganic compound preparationAcetic acidSulfite salt
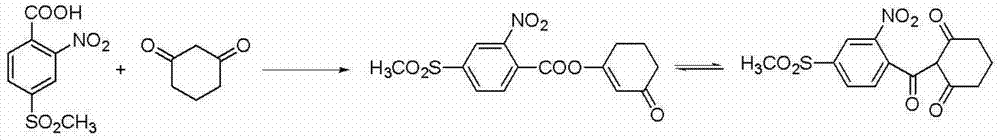
The invention provides a preparation method of mesotrione. The preparation method comprises is capable of preparing the target product from raw materials such as ortho-nitrotoluene, chlorosulfonic acid, sulfoxide chloride, sodium sulfite, chloroactic acid, 1,3-cyclohexanedione in the presence of a catalyst and acid-base. The preparation method is based on common chemical raw materials, the reaction flow of each step is implemented under conventional operation conditions, the amount of three wastes is low, the total yield is above 61%, the purity of the product is high, the purity of the coarse product of the mesotrione is greater than 98%, and the coarse product of the mesotrione can be further purified; as a result, the preparation method of mesotrione is applicable to large-scale industrial production.
Owner:INSIGHT FINECHEM +1
Synthesis method of indole
The indole synthesizing process includes the following steps: 1. the hydroxymethylation reaction of alkali catalyst, o-nitrotuluene, paraformaldehyde and polar solvent through stirring at 0-150 deg.c for 10 min to 5 hr, regulating pH and decompression fractionizing to obtain o-nitro phenethyl alcohol; 2. the catalytic hydrogenation reaction of o-nitro phenethyl alcohol with added skeletal nickel catalyst at 50-200 deg.c and 1-100 atm hydrogen pressure for 1-20 hr to obtain o-amino phenethyl alcohol; and 3. cyclization reaction of o-amino phenethyl alcohol with added metal modified skeletal catalyst at 110-300 deg.c for 1-10 hr to obtain indole. The present invention has facile cheap material, low cost of catalyst, short reaction time and simple indole synthesizing process, and the product indole has wide use and high economic value.
Owner:TIANJIN UNIV
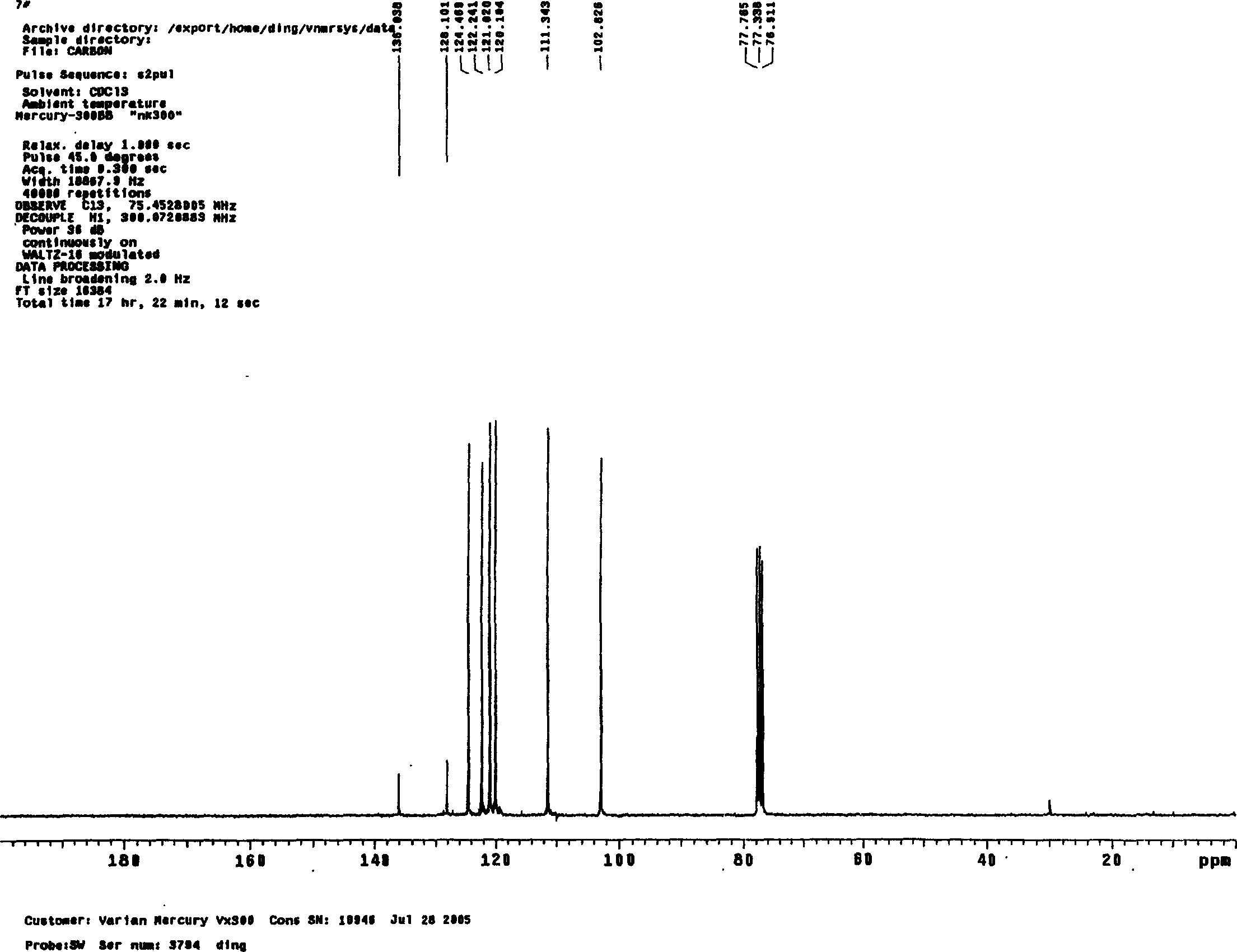
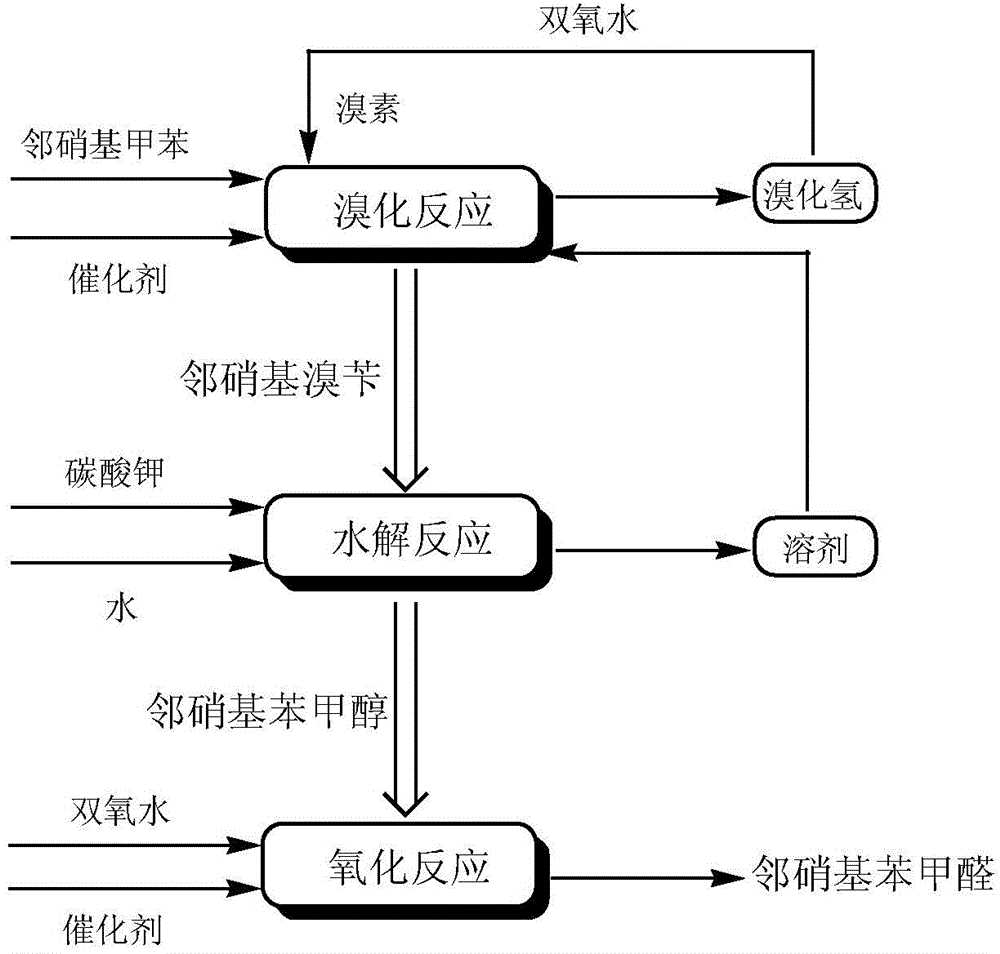
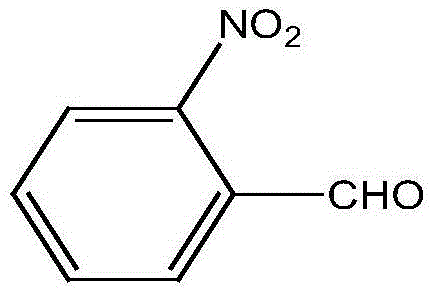
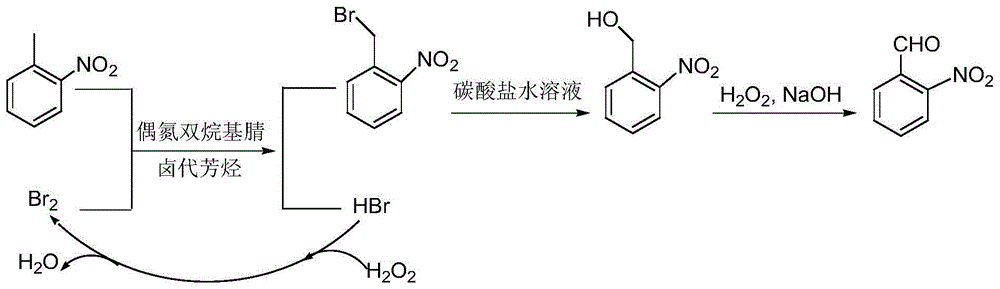
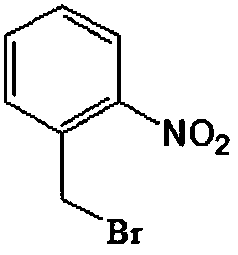
A preparing method of 2-nitrobenzaldehyde
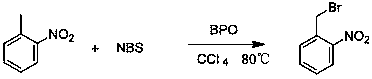
InactiveCN105439867AEasy to cleanReduce pollutionOrganic chemistryOrganic compound preparationCatalytic functionOperation safety
The invention discloses a preparing method of 2-nitrobenzaldehyde. 2-nitrotoluene is adopted as a raw material and is brominated with bromine under catalytic function of azo-bis alkyl nitrile to generate 2-nitrobenzyl bromide and hydrogen bromide. The 2-nitrobenzyl bromide is hydrolyzed under catalytic function of an aqueous carbonate solution to generate 2-nitrobenzyl alcohol. The 2-nitrobenzyl alcohol is oxidized with hydrogen peroxide under catalytic function of sodium hydroxide to generate the objective compound, namely the 2-nitrobenzaldehyde. A hydrogen peroxide oxidation manner is adopted by the method, thus improving cleanliness of industrial preparation reactions, and reducing environment pollution. Oxidation is catalyzed by adopting the inorganic solid alkali catalyst and no metal organic complex catalyst is used, thus improving reaction stability and greatly reducing the cost of industrial preparation. An azo-bis alkyl nitrile solid catalyst in place of a peroxydicarbonate liquid catalyst is adopted to catalyze the bromination, thus improving reaction operation safety of industrial preparation. The method increases the product yield. The yield of the method is increased by about 5% than that of traditional industrial methods at present. The total yield can reach 77% and product purity is higher than 99%.
Owner:NANJING UNIV OF SCI & TECH
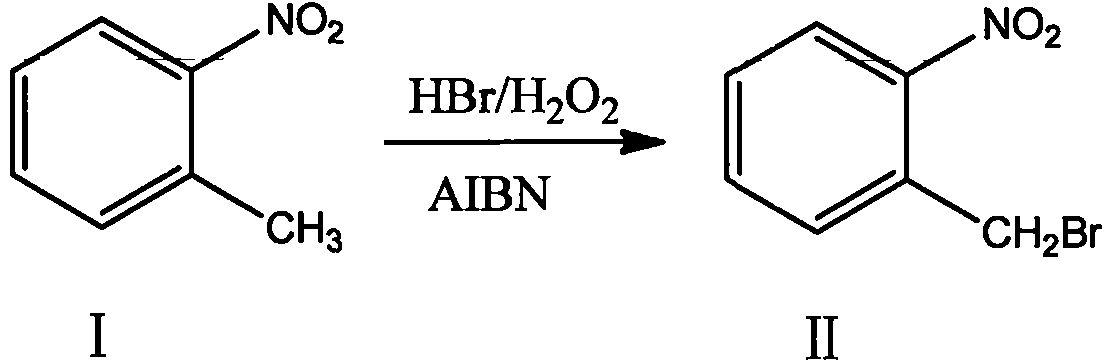
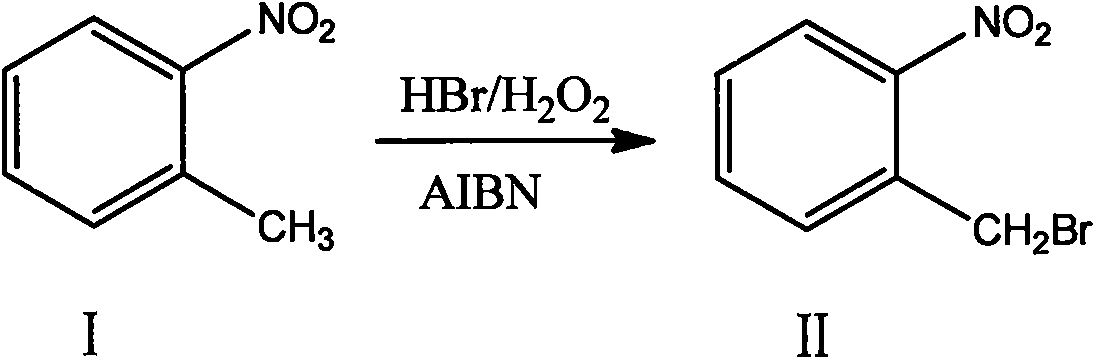
Production method for 2-nitrobenzyl bromide
ActiveCN103641722ASimple post-processingLow costOrganic chemistryOrganic compound preparationSulfite saltThermal insulation
The invention discloses a production method for 2-nitrobenzyl bromide. The method comprises the following steps: raw materials comprising 100kg of ortho-nitrotoluene, 110kg of hydrogen bromide with a mass concentration of 48%, 10kg of a catalyst azodiisobutyronitrile and 2kg of a phase transfer agent polyvinyl alcohol PEG600 are added into 250kg of water, the above mixture is stirred and heated up, 100kg of hydrogen peroxide with a mass concentration of 30% are added dropwisely and slowly at the temperature of 50-82 DEG C. After the addition, the mixture is subjected to a thermal insulation reaction for hours until the red color fades away. The upper layer water layer is separated, and the lower layer material layer is washed to be neutral with 5% of sodium sulfite, and then washed with water. With petroleum ether as a solvent, the synthetics are subjected to recrystallization and purification, and white crystal 2-nitrobenzyl bromide is obtained. Each material can be amplified according to the given weight ratios. No organic solvents are employed, the post-treatment is simple, and the cost is low. The reaction yield is above 79.4%, and is far higher than the yield 72% of traditional technology production.
Owner:SHANDONG XINGQIANG CHEM IND TECH RES INST CO LTD
Synthesis technology of pyraclostrobin
InactiveCN106008347AImprove selective reducibilityHigh yieldOrganic chemistryMicro nanoHydroxylamine
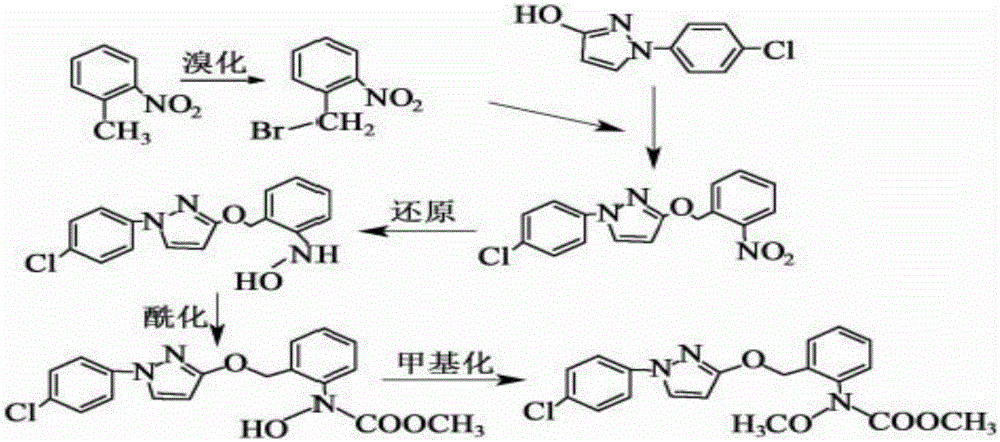
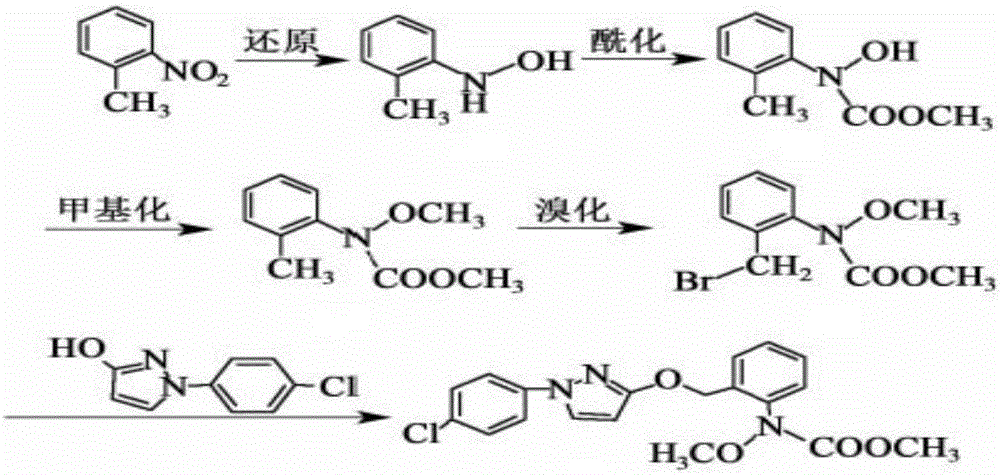
The invention provides a synthesis process of pyraclostrobin, which can be divided into five steps: (1) o-nitrotoluene and NH 4 Cl reduction reaction occurs under the catalysis of zinc powder and alloy micronano powder; (2) acylation reaction of hydroxylamine; (3) methylation reaction; (4) N-methoxy-N -2-bromomethylphenyl methyl carbamate; (5) use DMF as a solvent to dissolve N-methoxy-N-2-bromomethyl phenyl methyl carbamate and make a solution for subsequent use, and 1-( 4‑chlorophenyl)‑pyrazolol, K 2 CO 3 , acetone and put them into the reactor together, after heating up and refluxing, slowly add N-methoxy-N-2-bromomethylphenyl carbamate solution in the reactor, and the product pyrazole ether is obtained after the reflux reaction is completed Strostrobin. Compared with the prior art, the preparation method of the present invention is simple, the raw materials are cheap and easy to obtain, the reaction conditions are mild, and the purity and yield of the obtained target product are high.
Owner:ANHUI GUANGXIN AGROCHEM
Technology for synthesizing pyraclostrobin by six-step method
The invention provides a technology for synthesizing pyraclostrobin by a six-step method. The synthesis technology comprises the following steps: 1) performing a reduction reaction of ortho-nitrotoluene; 2) performing an acylation reaction of hydroxylamine; 3) performing a methylation reaction; 4) obtaining N-methoxyl-N-2-bromomethyl phenyl methyl carbamate through a bromination reaction; and 5) performing synthesis of 1-(4-chlorophenyl)pyrazolidine-3-ketone; and 6) performing synthesis of pyraclostrobin. Compared with the prior art, the preparation method has the advantages of simple process, easily available raw materials with low cost, and mild reaction condition, and the purity and yield of the target products are high.
Owner:ANHUI GUANGXIN AGROCHEM
Novel nitrobenzyl bromide synthesis process
ActiveCN107778181AHigh yieldHigh purityOrganic chemistryOrganic compound preparationBromineOxidizing agent
The invention relates to a novel nitrobenzyl bromide synthesis process which comprises the following steps: in the presence of 40% hydrobromic acid, 30% hydrogen peroxide and an initiator, namely azodiisobutyronitrile, by taking ortho-nitrotoluene as a raw material, performing a free radical bromination reaction, thereby synthesizing the nitrobenzyl bromide. The 30% hydrogen peroxide is adopted asan oxidant in the process, bromine is provided by oxidizing the hydrobromic acid, the testing conditions are strictly regulated and controlled, then the free radical bromination reaction of the ortho-nitrotoluene has selectivity, the synthesis procedures are controllable, no dibromo-byproduct is generated, meanwhile the yield is far greater than that of the prior art, the production cost is low,the environment is protected, and the process is an ideal method applicable to large-scale industrial production at present.
Owner:ZHEJIANG ZHONGSHAN CHEM IND GRP
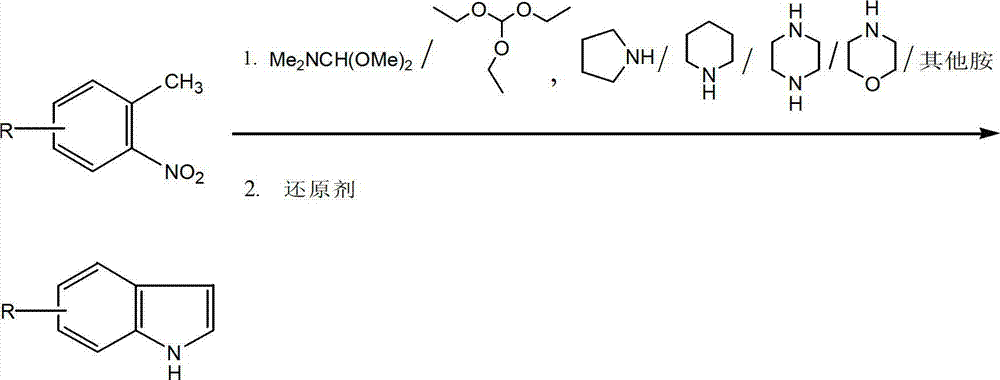
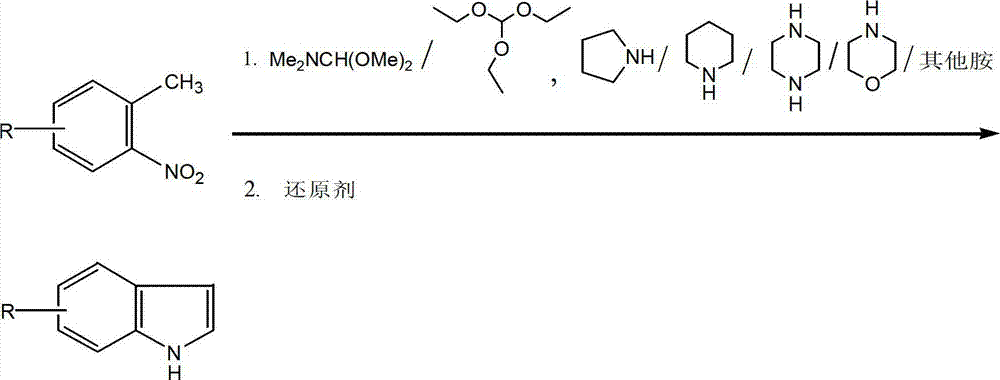
Method for synthesizing substituted indole compounds through one-pot method
The invention relates to a synthesis method of substituted indole compounds, and particularly relates to a method for synthesizing substituted indole compounds through a one-pot method. The method comprises the following steps: under alkaline and anaerobic conditions, reacting ortho-nitrotoluene derivatives and N,N-dimethylformamide dimethyl acetal or triethyl orthoformate used as raw materials in an organic solvent; and then, adding a reducer, and performing reduction and cyclization reaction to obtain indole derivatives, wherein R is a monosubstitution or polysubstitution located on site 4, 5, 6 or 7; and the R substituent is hydrogen, alkyl, substituted alkyl, alkoxy, amino or halogen atom. According to the invention, a one-pot method is adopted; the conventional and readily accessible ortho-nitrotoluene compounds are directly used as raw materials for reaction; separation and purification of intermediate compounds are not required; and the indole derivatives can be synthesized through the one-pot method by effectively controlling the reaction conditions, the charging sequence and the charging ratio. According to the invention, the technological operation procedure is simplified, the reaction time is shortened, the cost is saved, the total yield is improved, and better production and practical values can be achieved.
Owner:YANTAI INST OF COASTAL ZONE RES CHINESE ACAD OF SCI
Preparation method of L-octohydroindoline-2-formic acid
The invention discloses a preparation method of L-octohydroindoline-2-formic acid. The method comprises the following steps of: preparing indole-2-formic acid through condensation and hydrogenation by using diethyl oxalate and ortho-nitrotoluene as raw materials; adding acetic anhydride and hydrochloric acid by using the indole-2-formic acid as a raw material to carry out acylation and hydrogenation working procedures to obtain a (R, S) indoline-2-formic acid mixture; splitting the (R, S) indoline-2-formic acid mixture to obtain R indoline-2-formic acid; treating the R indoline-2-formic acid, water and hydrochloric acid through racemizing, cooling, regulating the pH value, centrifuging and drying to obtain S indoline-2-formic acid; and reacting the S indoline-2-formic acid, methanol and a catalyst to obtain the L-octohydroindoline-2-formic acid. The invention has the advantages of low cost, clean and environmentally-friendly hydrogenation route, less generated waste water and high yield of the final finished product L-octohydroindoline-2-formic acid.
Owner:安徽世华化工有限公司
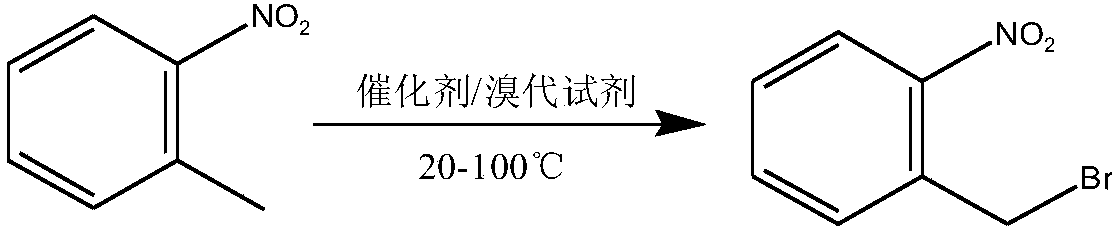
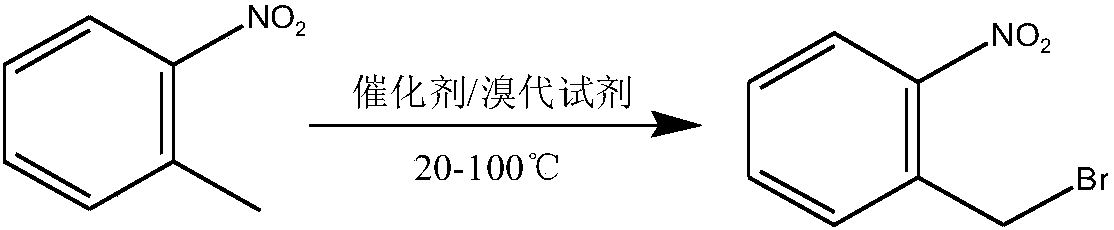
Method used for preparing o-nitrobenzyl bromide
ActiveCN108203384AHigh yieldIncrease profitOrganic chemistryOrganic compound preparationBromineReagent
The invention discloses a novel preparation method of o-nitrobenzyl bromide. According to the preparation method, ortho-nitrotoluene is taken as a raw material, azodiisobutyronitrile is taken as a catalyst, at 20 to 100 DEG C, a bromination reagent is adopted for bromination to prepare the target product. Compared with the prior art, the preparation method possesses following advantages: 1, yieldis increased obviously from about 60% to about 90%; 2, the bromination reagent can be recycled, and bromine element utilization rate is relatively high, is 90% or higher; 3, reactants can be subjectedto direct mixing reaction, so that operation complexity is reduced; and 4, reaction conditions are convenient to control, the post-treatment is simple, less three waste is generated, and the industrial using value is relatively high.
Owner:SHANGDONG HAILIER CHEM
Method for producing O-methyl phenyl hydroxylamine
ActiveCN104163775AThe reduction reaction is simple and easy to controlLow costOrganic chemistryHydroxylamineHydrazine compound
The invention discloses a method for producing O-methyl phenyl hydroxylamine. The method comprises the following steps: adding ortho-nitrotoluene, a solvent and a catalyst to a reaction kettle, and stirring the materials, wherein the weight ratio of the ortho-nitrotoluene to the solvent to the catalyst is (35-45) to (80-150) to (5-6); then dropwise adding hydrazine hydrate at 0-8 DEG C, wherein the weight ratio of the hydrazine hydrate to the catalyst is (20-50) to (5-6); carrying out heat preservation reaction at 2-10 DEG C for 4-6 hours after dropwise adding is ended; removing the catalyst by suction filtration after cooling, standing and separating a water layer, and then removing the solvent by vacuum rotary evaporation; and recrystallizing the product by using petroleum ether, carrying out suction filtration and drying, so as to obtain the O-methyl phenyl hydroxylamine. The method disclosed by the invention has the advantages that reduction reaction is simple and easy to control, complicated hydrogenation device is not required, the cost is low, a raney nickel catalyst is adopted, and the reaction yield can be up to over 85%, and is much higher than that of normal process production, which is 70%.
Owner:SHANDONG XINGQIANG CHEM IND TECH RES INST CO LTD
Preparation method of osimertinib intermediate
ActiveCN109485638AEmission reductionImprove protectionOrganic chemistryN dimethylformamideDimethyl acetal
The invention relates to a preparation method of an osimertinib intermediate, comprising: subjecting ortho-nitrotoluene and 3,3-dialkoxypropanenitrile as raw materials to alkali-catalyzed nucleophilicaddition to obtain 1-(2-nitro)phenyl-4,4-dialkoxy-2-butanone, subjecting 1-(2-nitro)phenyl-4,4-dialkoxy-2-butanone and N,N-dimethylformamide dimethyl acetal to thermal condensation to obtain 1-dimethylamino-2-(2-nitro)phenyl-5,5-dialkoxy-3-n-pentanone, subjecting the attained reaction liquid to direct catalytic hydrogenation to obtain 3-(3,3-dialkoxy)propionylindole, subjecting 3-(3,3-dialkoxy)propionylindole to reaction with a methylation reagent under alkaline conditions to generate 3-(3,3-dialkoxy)propionyl-N-methylindole, and subjecting 3-(3,3-dialkoxy)propionyl-N-methylindole and 2-methoxy-4-fluoro-5-nitrophenylguanidine to exocondensation to obtain the osimertinib intermediate. The materials herein are low in price and easy to attain, the route is short, and the preparation method is environmentally friendly and high in yield.
Owner:XINFA PHARMA
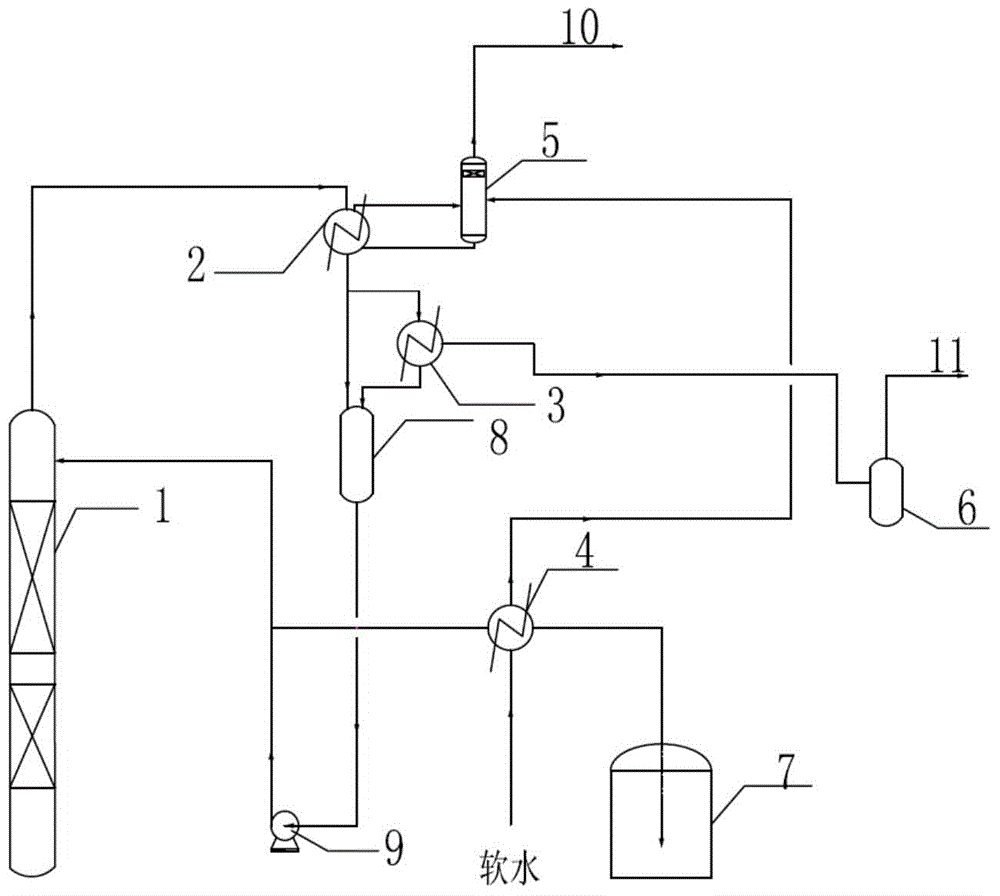
Method for by-producing steam in ortho-nitrotoluene separation process
InactiveCN104447343AEfficient use ofReduce pollutionOrganic chemistryOrganic compound preparationGas phaseOrtho position
The invention relates to a method for by-producing steam in the ortho-nitrotoluene separation process. The method comprises the steps of feeding a material into an adjacent rectifying column for rectifying; enabling the product vapor phase to flow from the top of the adjacent rectifying column into a condenser to be condensed; transferring the non-condensed product vapor phase into a sub-cooler for cooling; feeding the cooled product and the product condensed by the condenser into a reflowing tank; enabling the non-condensed trace vapor phase to flow from the sub-cooler into a vacuum collecting system through a vacuum buffering tank to be discharged; transferring part of the material from the reflowing tank into the adjacent rectifying column through a reflowing pump for reflowing, and processing the rest part of the material by cooling and then feeding into a product storage tank, wherein the product before being fed into the product storage tank is cooled through a soft water preheater, the soft water is preheated at the same time, then the preheated soft water enters a steam drum, saturated water in the steam drum enters the condenser to be used as a refrigerant medium, and the produced saturated steam is returned to the steam drum and then enters a steam pipe network after gas-liquid separation. The method is simple in process, the latent heat produced by cooling the product can be effectively utilized to produce steam, and therefore, the cost is decreased, the energy is saved, and the environment is protected.
Owner:QINGDAO UNIV OF SCI & TECH +2
Synthetic method of 4-fluorobenzaldehyde
InactiveCN101353297ALow priceShort synthetic routeOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSulfolaneHexamethylphosphoramide
The invention relates to a synthetic method of p-flurobenzaldehyde, which belongs to the chemical and pharmaceutical field. In the synthetic method, the p-chlorobenzaldehyde and potassium fluoride react under the condition of a solvent and a catalyst at high temperature, wherein, the solvent is one of sulfolane, dimethyl sulfoxide, dimethylformamide, dimethylacetamide, hexamethyl phosphoramide, nitrobenzene and ortho-nitrotoluene; the catalyst is one of or the mixture of two or more than two of benzyl triethyl amine chloride, tetrabutylammonium bromide, hexadecyltrimethylammonium chloride, methyltrioctylammonium chloride, tetraphenylphosphonium bromide, methyltriphenylphosphonium bromide, benzyl triphenyl phosphonium bromide and polyethylene glycol dimethyl ether. Compared with the conventional preparation method, the synthetic method of the invention has the advantages of cheap raw materials, short synthetic route and little 'three wastes' discharge. As the amount of the catalyst used is reduced and the price of the solvent is low, the production cost of the p-flurobenzaldehyde is decreased.
Owner:王俊华
Preparation method of nitrobenzyl bromide
ActiveCN108069860AReduce contentSimple and fast operationOrganic chemistryOrganic compound preparationHalohydrocarbonHydrogen bromide
The invention discloses a preparation method of nitrobenzyl bromide. The preparation method comprises the following steps of: step 1, dissolving ortho-nitrotoluene in halohydrocarbon, sequentially adding a hydrogen bromide solution, an initiator and lewis acid, continuing stirring and heating for backflow reaction, and step 2, dropwise adding a hydrogen peroxide solution into a reaction system instep 1 at a speed of 1-2 drops per min, after the backflow reaction for 1-3h, performing natural cooling, stopping stirring, and performing separation to form an organic phase, namely nitrobenzyl bromide. The method takes ortho-nitrotoluene as a raw material and hydrogen bromide as a bromine source; the hydrogen peroxide solution is added as an oxidant; the initiator and lewis acid are added for ortho-nitrotoluene bromination reaction to generate nitrobenzyl bromide; the preparation method is easy and simple to operate and high in yield; and a content of a byproduct, namely o-nitrocyclite is low.
Owner:SOUTHEAST UNIV
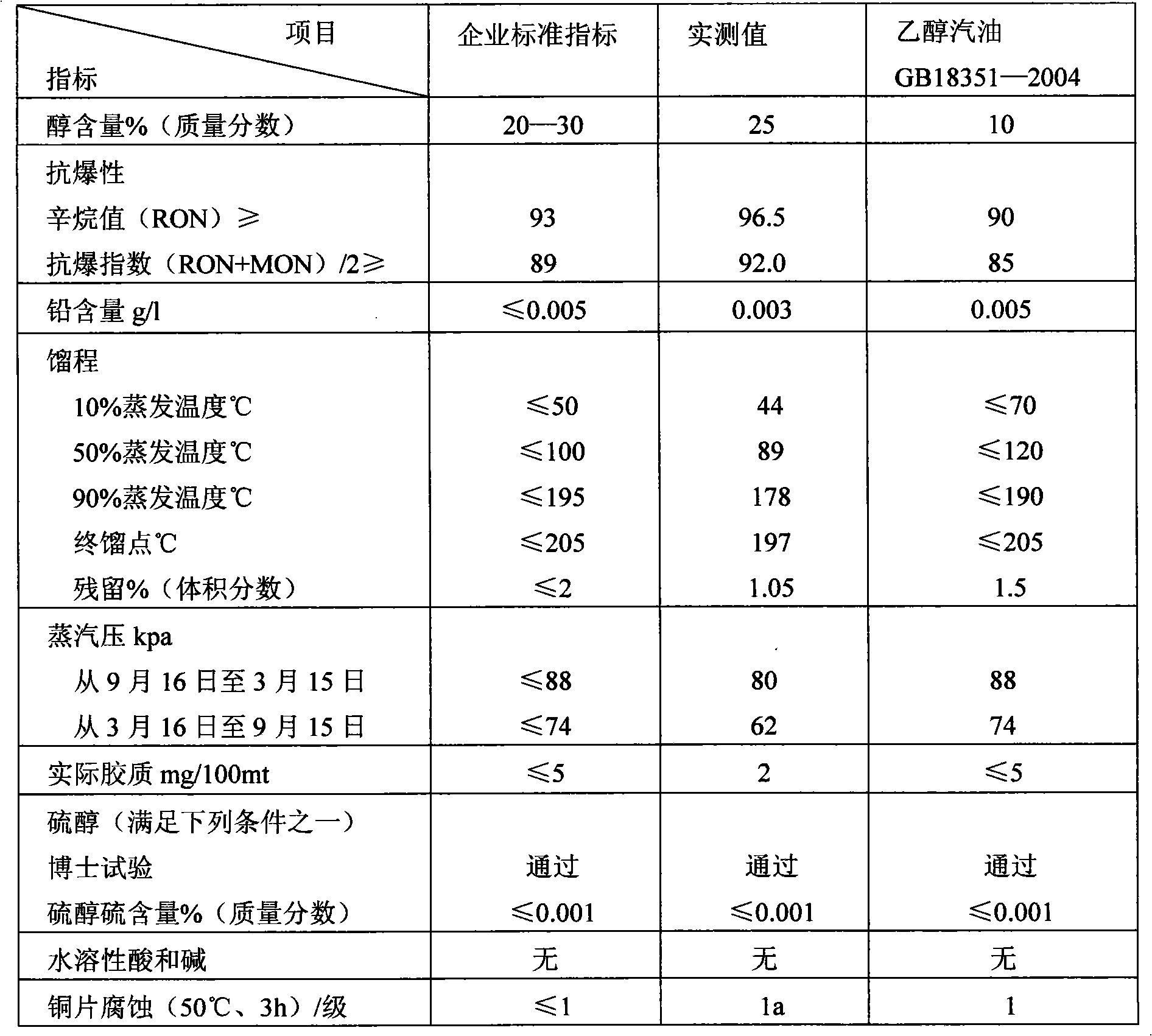
Low-carbon denatured alcohol gasoline and application
InactiveCN101597523ALow costSave resourcesLiquid carbonaceous fuelsFuel additivesDenatured alcoholMethanol fuel
The invention discloses a low-carbon denatured alcohol gasoline and application. The low-carbon denatured alcohol gasoline comprises the following components in percentage by weight: 60-80% of alcohol, 5-15% of fuel ethanol and 5-26% of additive, wherein the additive is composed of the following components in percentage by weight: 45-55% of ortho-nitrotoluene, 20-37% of dimethoxymethane, 5-8% of dimethyl malenate, 0.4-.06% of corrosion inhibitor for metal and 0.4% of antiswelling inhibitor for rubber. The low-carbon denatured alcohol gasoline is added into the national standard gasoline in percentage by weight of 20-85% to be applied. The low-carbon denatured alcohol gasoline is a motor gasoline with better cleanability, lower gasoline consumption rate, larger mixing amount and lower raw material cost; the cost of the motor gasoline is obviously lowered, resource is saved, and engine emission is further improved; at the same time, the motor gasoline solves the problems of high gasoline consumption, low-temperature layering, and the like, lowers saturation vapour pressure and prevents the air blocking phenomenon.
Owner:莫春福
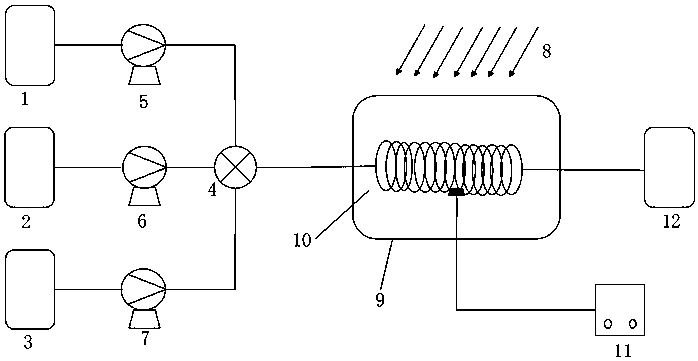
Device for continuous preparation of o-nitrobenzyl bromide and preparation method thereof
InactiveCN109999747AShort reaction timeReduce backmixingOrganic compound preparationOrganic chemistry methodsMeasuring instrumentOrganic layer
The invention relates to a device for continuous preparation of o-nitrobenzyl bromide and a preparation method thereof. The device mainly comprises a tubular reactor, three storage tanks, three corresponding metering pumps, an efficient mixer and a receiving tank. The storage tanks are connected to the efficient mixer through pipelines. The metering pumps are distributed on the connecting pipelines of the corresponding storage tanks and the efficient mixer. The efficient mixer is successively connected to the tubular reactor and the receiving tank. A light source is arranged above the tubularreactor. A temperature measuring instrument is arranged in the tubular reactor. The preparation method comprises the following steps: raw materials including ortho-nitrotoluene, liquid bromine and water enter the mixer respectively through the three metering pumps to be uniformly mixed; the mixture then enters a pre-insulated tubular reactor to react under illumination; the reaction product entersthe receiving tank after the reaction, followed by standing; and an organic layer is taken, and an organic solvent is removed to obtain the product. The preparation method of o-nitrobenzyl bromide isconvenient to operate; the post-treatment is simple; the product has good yield and high purity, and is suitable for industrial production.
Owner:ZHEJIANG UNIV OF TECH
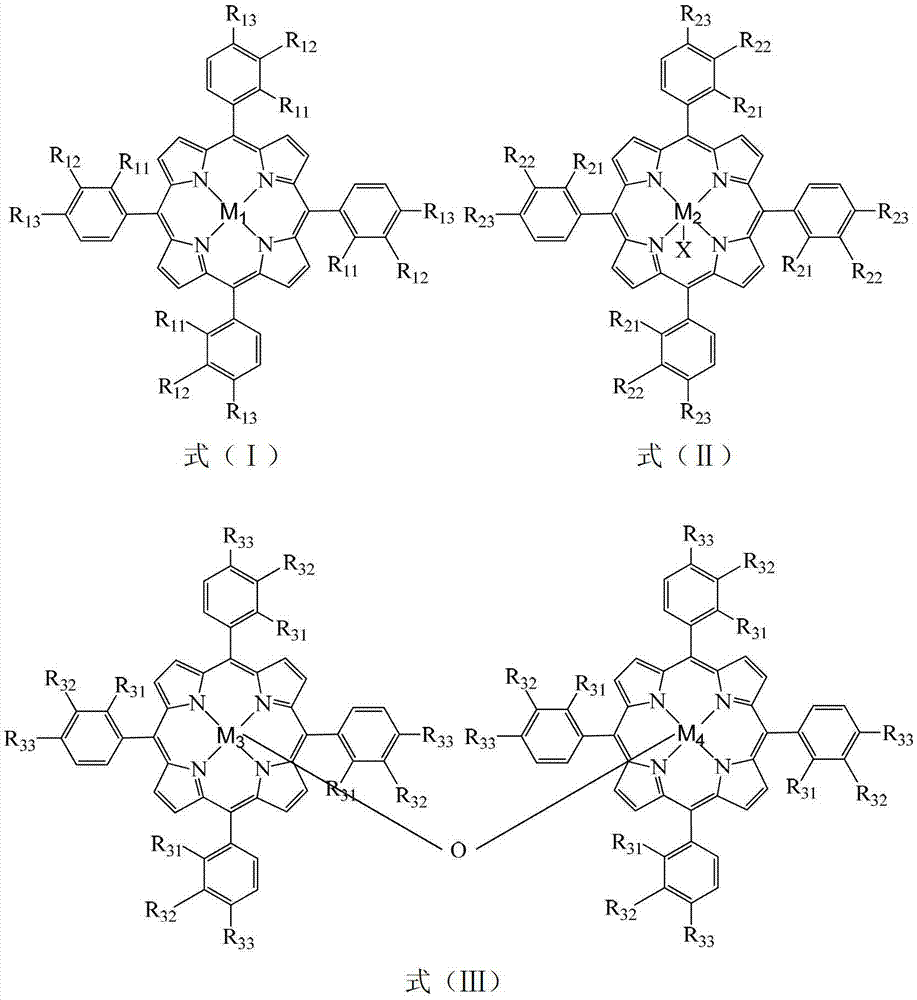
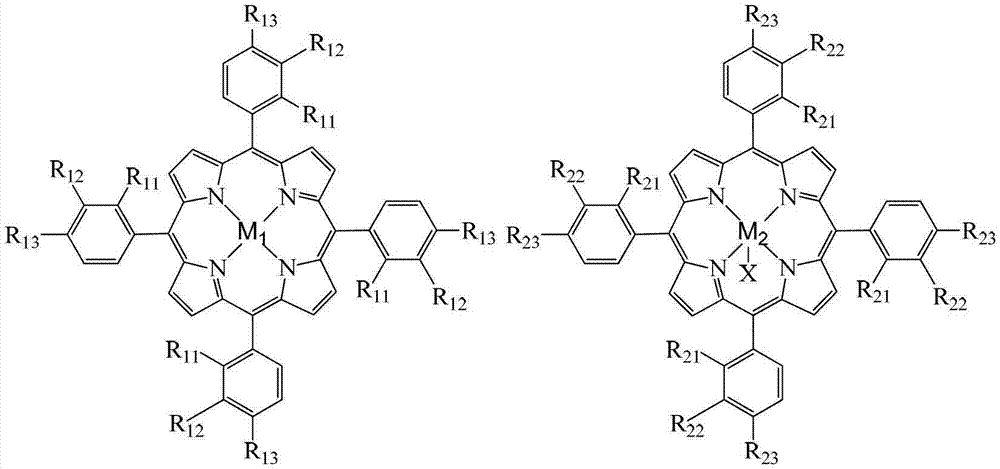
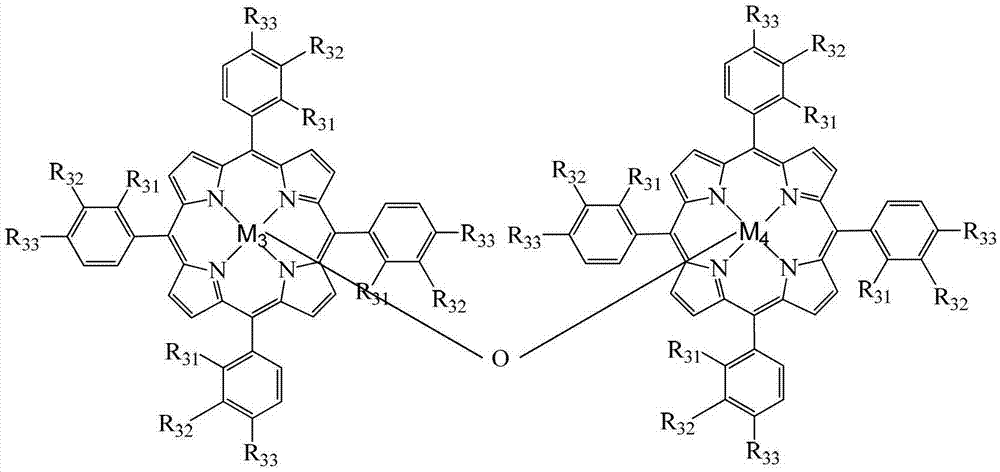
Method for preparing ortho-hydroxybenzoic acid by catalyzing and oxidizing ortho-nitrotoluene with metalloporphyrin and metal salt compound as catalyst
InactiveCN103193654APromote reciprocal activationEfficient use ofOrganic chemistryOrganic compound preparationPorphyrinOxygen
The invention relates to a method for preparing ortho-hydroxybenzoic acid by catalyzing and oxidizing ortho-nitrotoluene with a metalloporphyrin and metal salt compound as a catalyst. The method comprises the following steps of: introducing the oxygen of 0.5-2.5Mpa into the aqueous ethanol of 0.5-2.5mol / L sodium hydroxide by taking the aqueous ethanol comprising 50-95% by volume of ethanol as a solvent and taking the compound of a metalloporphyrin and a metal salt as a catalyst, or the compound of any two or three of a metalloporphyrin of formula I, a metalloporphyrin of formula II and a metalloporphyrin of formula III as a catalyst, or the compound of a metalloporphyrin of formula I and a metalloporphyrin of formula I or a metalloporphyrin of formula II and a metalloporphyrin of formula II or a metalloporphyrin of formula III and a metalloporphyrin of formula III as a catalyst, and carrying out reaction at the temperature of 35-55 DEG C for 1-6 hours to obtain the ortho-hydroxybenzoic acid, wherein the concatenation of the metalloporphyrin is 5-50ppm, and the concentration of the metal salt is 5-200ppm. The method provided by the invention has the advantages of high target product yield, short reaction time, small use amount of the alkali and low toxicity of the solvent. Due to the adoption of the method, the resource can be saved effectively, the pollution to the environment can be reduced, and the purposes of comprehensively saving energy and reducing emission can be achieved.
Owner:BEIJING UNIV OF TECH
Synthetic method for preparing pyraclostrobin intermediate from o-nitrobenzyl chloride
InactiveCN105859625AExcellent synthetic stepsThe synthesis steps are simpleOrganic chemistryNitrobenzeneChloride
The invention discloses a synthetic method for preparing pyraclostrobin intermediate from o-nitrobenzyl chloride, which comprises the following steps: adding a catalyst into o-nitrotoluene, heating and stirring, and slowly introducing chlorine gas, and after the reaction is finished, , reclaim o-nitrotoluene to obtain o-nitrobenzyl chloride; carry out etherification reaction with o-nitrobenzyl chloride and 1-(4-chlorophenyl)-3-pyrazolol to obtain 2-[(N- 4-chlorophenyl)-3-pyrazolyloxymethyl]nitrobenzene. The present invention replaces o-nitrobenzyl bromide with o-nitrobenzyl chloride and reacts with 1-(4-chlorophenyl)-3-pyrazolol to prepare pyraclostrobin intermediate 2-[(N-4 -Chlorophenyl)-3-pyrazolyloxymethyl]nitrobenzene, which avoids the defects of the existing synthetic method using o-nitrobenzyl bromide, and can prepare ethers with a content of ≥98% and a yield of ≥90%. chemical products.
Owner:SICHUAN FOURSTAR BIOTECH RANDD CORP
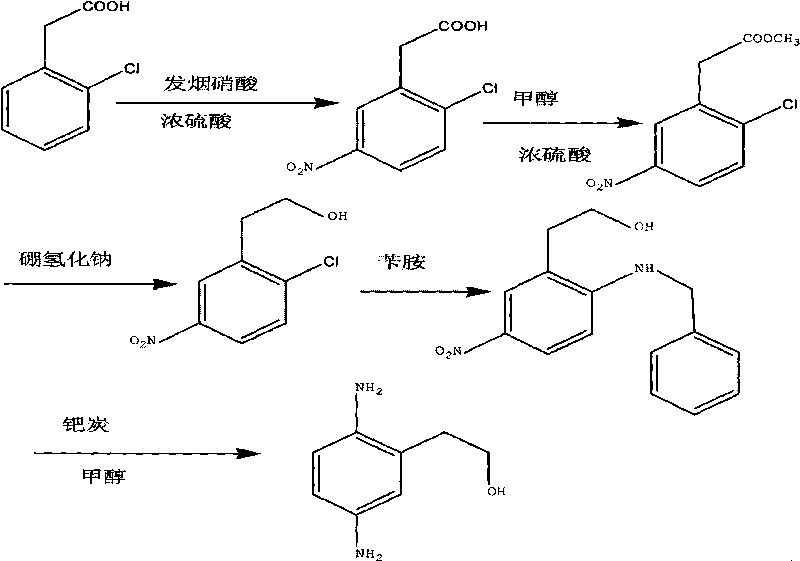
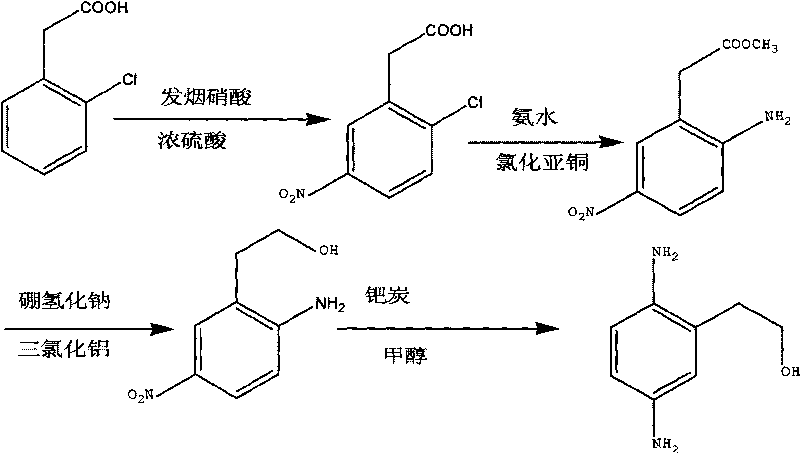
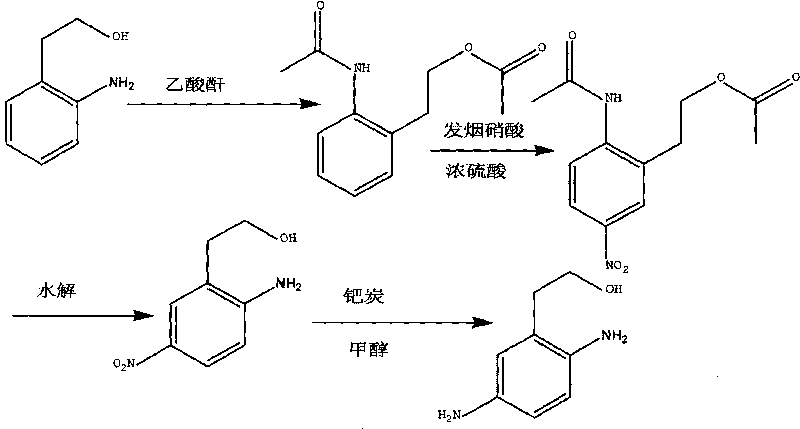
Synthesis method of 2,5-diamino benzene ethanol
ActiveCN101698647ARaw materials are easy to obtainMild reaction conditionsOrganic compound preparationAmino-hyroxy compound preparationSynthesis methodsNitration
The invention discloses a synthesis method of 2,5-diamino benzene ethanol, which takes ortho-nitrotoluene as a starting material, and 2,5-diamino benzene ethanol is obtained by the processes of carboxylation, acetylation, nitration, esterification, reduction, hydrolyzation, hydrogenation and the like and the process of salifying with sulfuric acid finally. The process route has the advantages of easily obtained raw materials, mild reaction condition, high and stable yield, low cost, simple operation and the like, thereby being applicable to industrial production.
Owner:辽宁新宇生物科技有限公司
Preparation method of o-nitrobenzyl bromide
InactiveCN109369407ARaw materials are easy to getLow costOrganic chemistryOrganic compound preparationRaw materialBromide
The invention discloses a preparation method of o-nitrobenzyl bromide. The preparation method comprises the steps that ortho-nitrotoluene is taken as a raw material, NaClO / HBr is taken as a bromatingagent, and the o-nitrobenzyl bromide is prepared through a bromating reaction under the initiation effect of azodiisobutyronitrile serving as an initiator. According to the preparation method, the NaClO / HBr is taken as the bromating agent to prepare the o-nitrobenzyl bromide, raw materials are easy obtain and low in cost, the technology is easy, convenient and easy to operate, the reaction condition is mild, reaction selectivity is high, the reaction yield is greater than 72% (by ortho-nitrotoluene), the content of the o-nitrobenzyl bromide in a purified solid product is greater than 97%, andthe preparation method is suitable for being popularized in an industrialization mode.
Owner:HUNAN HAILI CHEM IND
Preparation method of o-methyl phenyl hydroxylamine
The invention discloses a preparation method of o-methyl phenyl hydroxylamine. The preparation method includes: using ortho nitrotoluene as a raw material, hydrazine hydrate as reductant, a ZSM-5 molecular sieve loaded with metal Ni as a catalyst to synthesize o-methyl phenyl hydroxylamine. Reaction yield can reach 95.0%, and byproducts are lower than 0.1%. The ZSM-5 molecular sieve treated with Fe3+ is adopted, Ni is loaded, the catalyst is an environment-friendly catalyst which is short in reaction period, high in product quality and high in yield, discharge of three wastes is reduced, requirements of environment-friendly chemical process is met, and the preparation method has good industrial application value.
Owner:ANHUI COSTAR BIOCHEM CO LTD
Preparation method for N-2-methylphenylhydroxyamine
InactiveCN106187810AEvenly dispersedLarge specific surface areaOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsHydrazine compoundReduced properties
The invention discloses a preparation method for N-2-methylphenylhydroxyamine, and belongs to the technical field of compound synthesis. According to the preparation method, the N-2-methylphenylhydroxyamine is synthesized by taking ortho-nitrotoluene as a raw material, taking hydrazine hydrate as a reducing agent, and taking a nano-nickel / gamma-Al2O3 material as a catalyst; the reaction yield can reach 98.0 percent or higher, and by-products are equal to or lower than 0.1 percent. The nano-nickel / gamma-Al2O3 material adopted by the preparation method has extremely strong reducing property; the catalytic activity and the selectivity are much higher than those of a conventional raney nickel catalyst; moreover, the catalyst is environment-friendly, short in reaction period, high in product quality and high in yield, can be applied repeatedly, reduces the emission of waste gas, waste water and waste residues, meets the requirement on green chemical process, and has a very industrial application value.
Owner:ANHUI COSTAR BIOCHEM CO LTD
Energy-saving fuel additive
InactiveCN106010688AImprove combustion efficiencyEffective chemistryLiquid carbonaceous fuelsCombustion chamberN-Propyl alcohol
The invention relates to an energy-saving fuel additive. The energy-saving fuel additive is prepared from, by mass, 30-35 parts of oxidized petrolatum, 20-26 parts of n-nonylphenol, 23-26 parts of glyceric acid ester, 15-20 parts of nonylphenol polyether amine, 6-12 parts of ortho-nitrotoluene, 3-8 parts of dinitrogen ethane, 12-15 parts of n-propyl alcohol and 60-70 parts of deionized water. By adoption of a special polyether amine washing technology, the energy-saving fuel additive has an effective chemical effect for integral fuel system clearing, is applicable to new direct-injection fuel systems and can be used in any types of engines such as gasoline and diesel engines to remove precipitates from combustion chambers and air inlet valves. After the energy-saving fuel additive is poured into a fuel tank, halting is effectively prevented, fuel combustion efficiency and horsepower can be improved, and emission reduction is realized.
Owner:洪其祥
Multifunctional fuel additive
InactiveCN105925324AImprove combustion efficiencyImprove horsepowerLiquid carbonaceous fuelsFuel additivesCombustion chamberOil additive
The invention relates to a multifunctional fuel additive. The multifunctional fuel additive is prepared from, by mass, 30-35 parts of oxidized petrolatum, 15-25 parts of naphtha, 12-18 parts of monostearate, 12-20 parts of polyol ester, 3-9 parts of ortho-nitrotoluene, 5-9 parts of nitrogen oxide and 60-70 parts of deionized water. The multifunctional fuel additive has an effective chemical action, is used for cleaning of a whole fuel system, is suitable for a novel direct-injection fuel system, can be used in any type of engines, such as gasoline engines and diesel engines, removes the sediments from a combustion chamber and an intake valve, is only needed to be poured into a fuel tank, effectively prevents pauses, improves fuel combustion efficiency and power and reduces emissions.
Owner:洪其祥
Method for preparing methylaniline by continuous catalytic hydrogenation reduction of nitrotoluene through liquid phase
InactiveCN108997136ALow costReduce lossOrganic compound preparationAmino compound preparationMethylanilineNano catalyst
The invention discloses a method for preparing methylaniline by continuous catalytic hydrogenation reduction of nitrotoluene through a liquid phase. The method comprises the following steps that: S1,the preparing stage: ortho-nitrotoluene, meta-methylnitrobenzene, paranitrotoluene and a nano-catalyst are weighed, the mass ratio of nitrotoluene to methylaniline is 4:10, the addition quantity of the nano-catalyst is 10% of the addition quantity of the nitrotoluene; S2: the starting stage: the well weighed ortho-nitrotoluene, meta-methylnitrobenzene, paranitrotoluene and the nano-catalyst are added into a high pressure reaction kettle, and a hydrogen steel cylinder reducing valve and a pressure regulating valve are opened. According to the method for preparing the methylaniline by continuouscatalytic hydrogenation reduction of the nitrotoluene through the liquid phase, the ortho-nitrotoluene, the meta-methylnitrobenzene and the paranitrotoluene replace a solvent methyl alcohol or ethylalcohol, the loss in the reaction process is reduced, the explosion easily caused by the excessively large internal pressure is prevented, meanwhile, the nano-catalyst replaces a skeleton Ni, the costof the material is reduced, and the characteristics of recycling and stability of the nano-catalyst are used.
Owner:麻城市天恒商贸有限公司
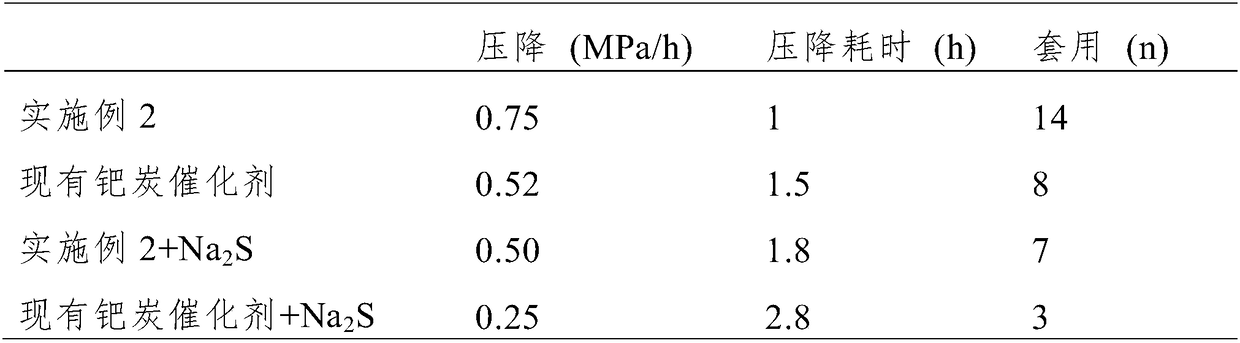
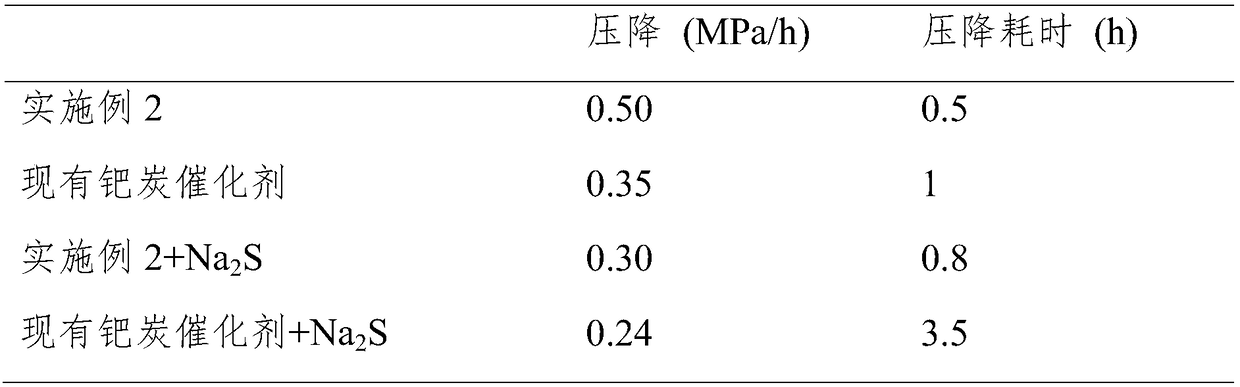
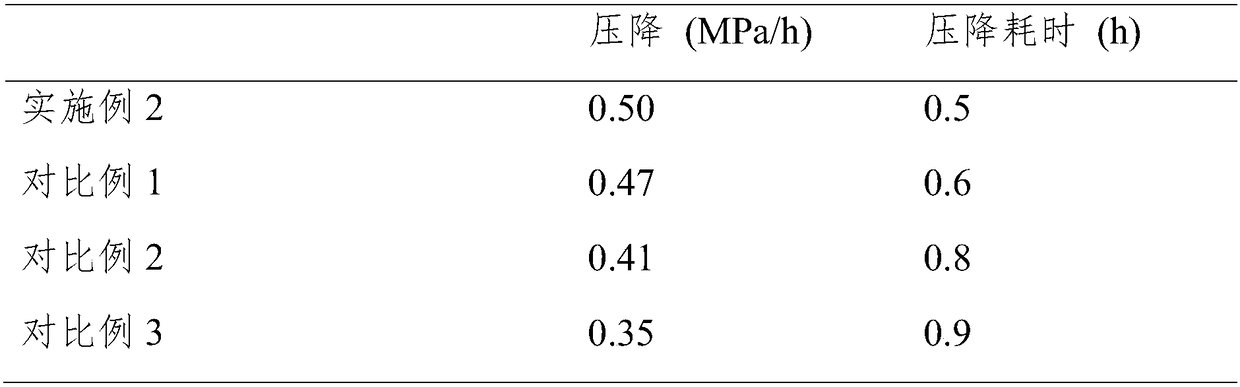
Preparation method of high-activity anti-sulfur-poisoning palladium carbon catalyst
InactiveCN109317144AImprove catalytic performanceImprove stabilityOrganic compound preparationAmino compound preparationActivated carbonFiltration
The invention discloses a preparation method of a high-activity anti-sulfur-poisoning palladium carbon catalyst. The preparation method includes the following steps: step 1, adding activated carbon into hydrochloric acid for cooking, performing filtering, adding solids obtained by filtration into nitric acid for cooking, and performing filtering and washing to obtain the acid-treated activated carbon; step 2, adding the acid-treated activated carbon into a modifier solution, and stirring the solution to obtain paste; step 3, adding the paste into an assistant metal salt solution, and stirringthe solution to obtain an activated carbon solution loaded with assistants; step 4, adding the activated carbon solution loaded with the assistants into a palladium chloride solution, adjusting the pH, stirring the solution to obtain a palladium suspension; step 5, reducing and filtering the palladium suspension, washing solids obtained by filtration, and centrifuging and spin-drying the solids toobtain the high-activity anti-sulfur-poisoning palladium carbon catalyst. The preparation method has the advantages that metal on the carrier surface of the obtained palladium carbon catalyst is highly dispersed, and high catalytic activity and anti-sulfur stability in hydrogenation of nitrobenzyl aniline and ortho-nitrotoluene are achieved.
Owner:XIAN CATALYST NEW MATERIALS CO LTD
Preparation method of 2-amino-4'-fluoro-diphenyl ketone
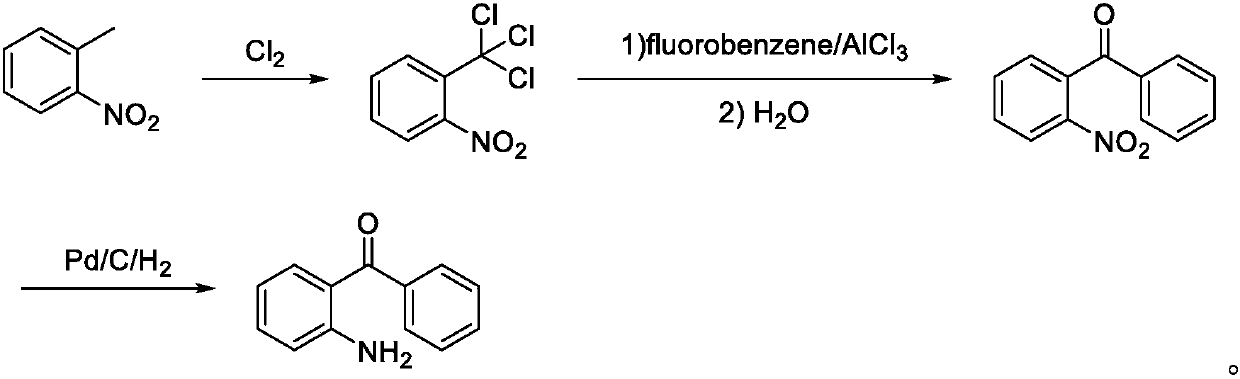
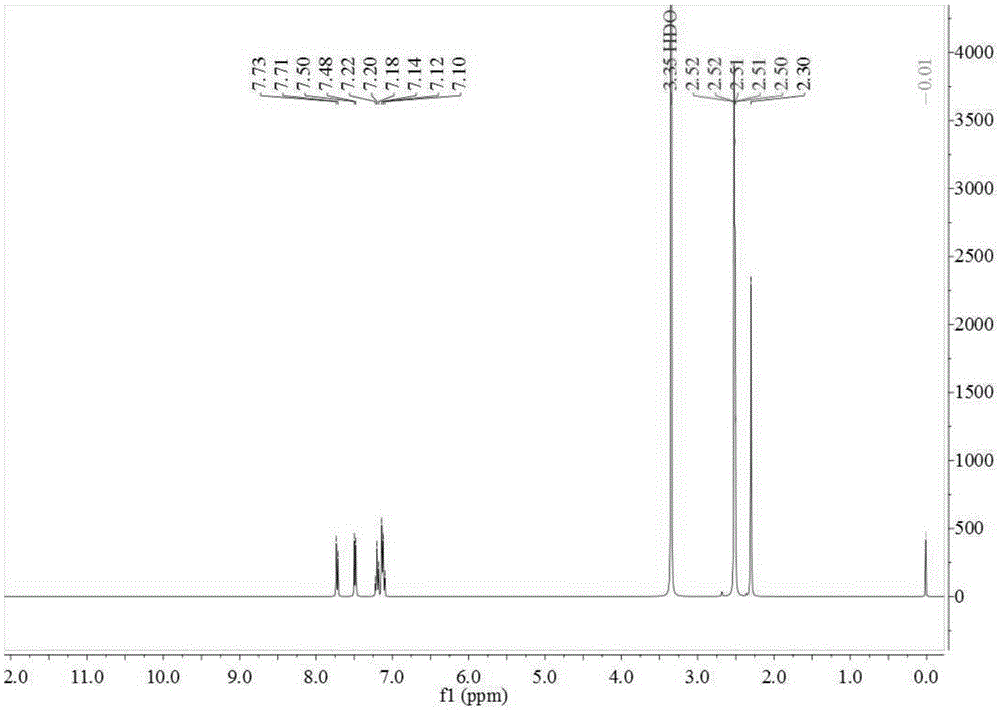
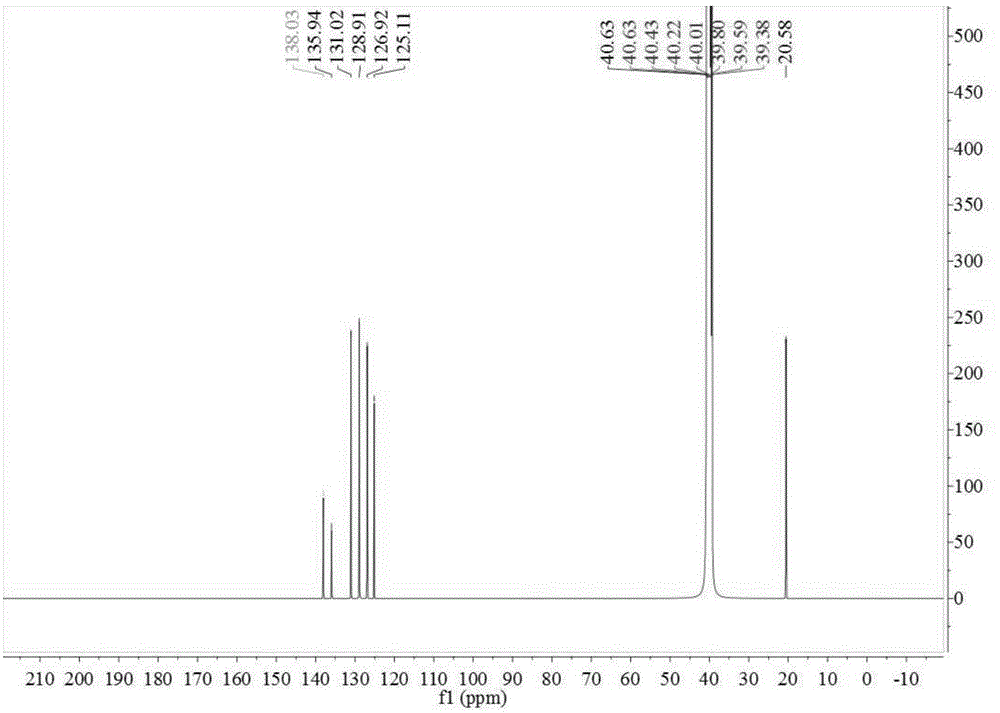
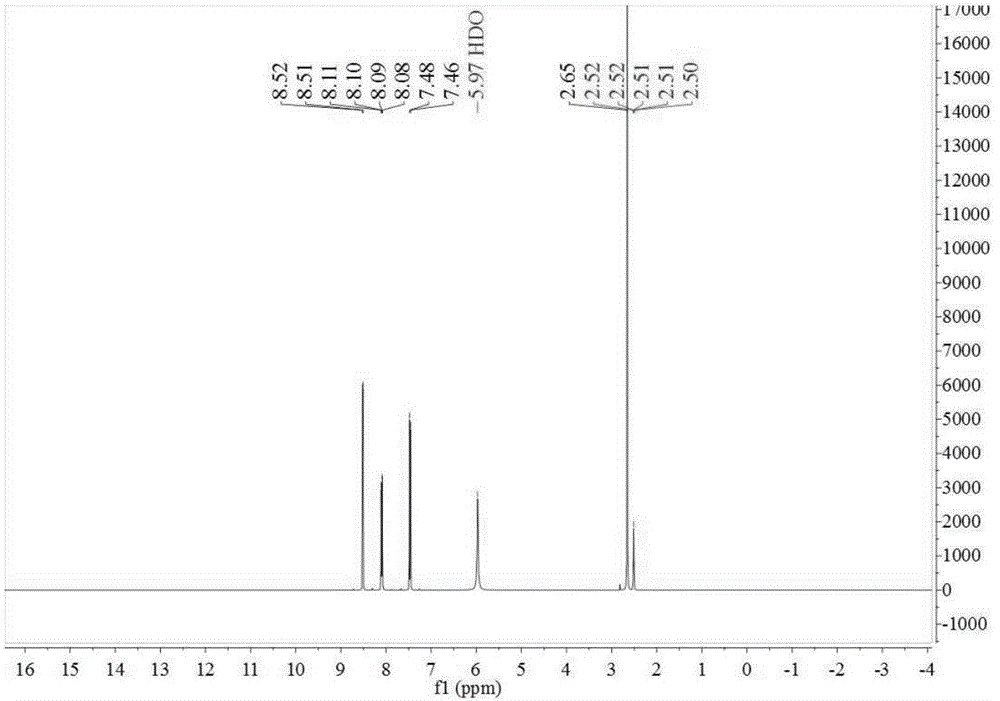
ActiveCN107673982AHigh yieldHigh purityOrganic chemistryOrganic compound preparationTolueneFluorobenzene
The invention discloses a preparation method of 2-amino-4'-fluoro-diphenyl ketone. The preparation method comprises the following steps that ortho-nitrotoluene is chloridized by chlorine gas to obtainO-nitrotrichlorotoluene; then the O-nitrotrichlorotoluene performs Fridel-Crafts hydrolysis reaction with fluorobenzene to obtain 2-nitryl-4'-fluoro-diphenyl ketone; the 2-amino-4'-fluoro-diphenyl ketone is obtained through reduction. The synthetic route of the method is green and friend to the environment, the yield is high, the prepared 2-amino-4'-fluoro-diphenyl ketone is high in purity, the starting raw materials are cheap and easy to obtain, and the method is low in cost, easy to operate and suitable for industrial production.
Owner:ANHUI QINGYUN PHARMA & CHEM
DSD acid preparation method
ActiveCN106146357AIncrease profitReduce formationSulfonic acid preparationChemical synthesisWhitening Agents
The invention relates to the field of chemical synthesis, in particular to a DSD acid preparation method. The DSD acid preparation method includes the steps that methylbenzene is subjected to sulfonation, purification and separation to obtain OTS, wherein the byproduct is PTS; OTS is subjected to mixed acid nitration to obtain PNTS; PNTS is subjected to chlorine oxidative condensation to obtain DNS; DNS is subjected to catalyzed hydrogeneration reduction to obtain the high-quality target product, namely DSD acid which can be directly used for synthesizing a fluorescent whitening agent. The synthesis method greatly reduces dangerousness of the process, greatly reduces generation of harmful byproducts and waste, particularly, does not generate a lot of carcinogenic intermediate, namely ortho-nitrotoluene, completely solves the nitration safety problem of the old technology, and has the advantages of being simple in step, high in yield and the like.
Owner:上海合丽亚日化技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com