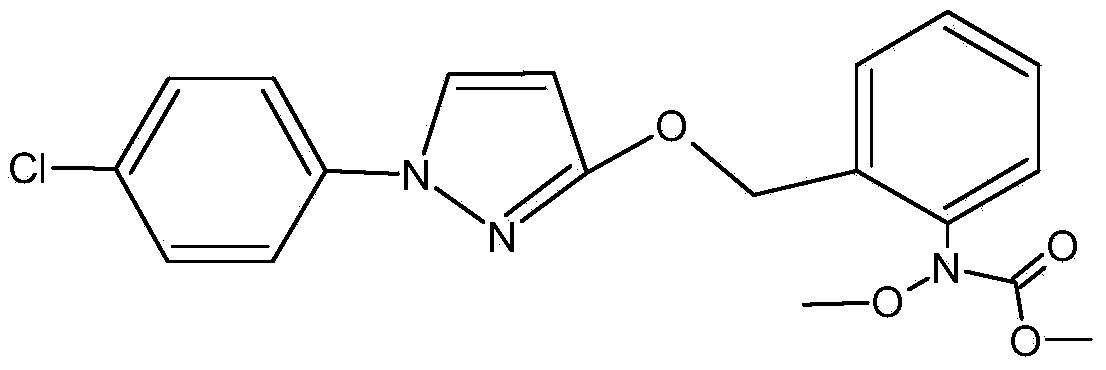
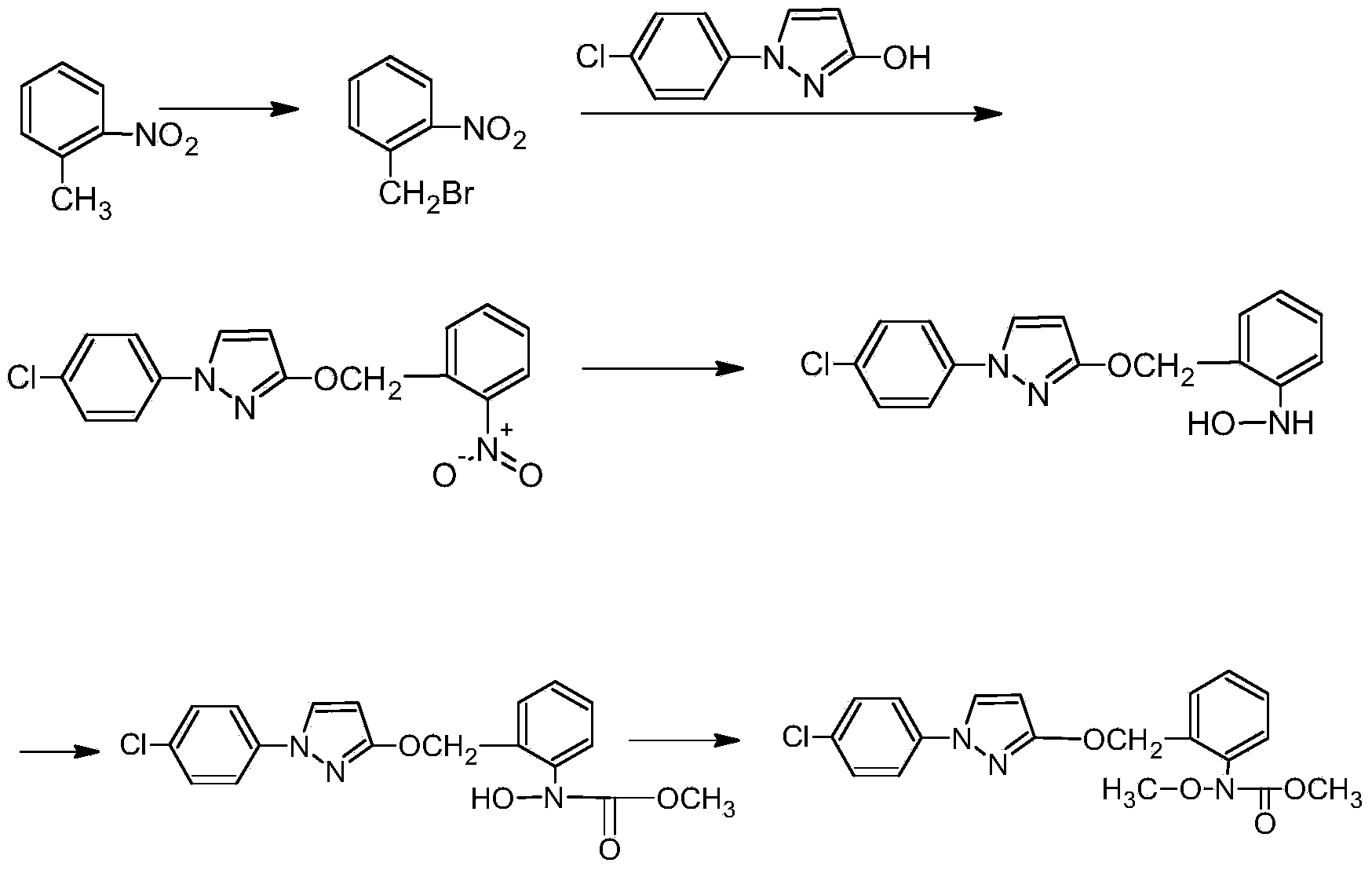
Method for producing O-methyl phenyl hydroxylamine
A technology of o-methylphenylhydroxylamine and methylphenylhydroxylamine, applied in the production field of o-methylphenylhydroxylamine, can solve the problems of high preparation cost, low bromination yield, restricting the application of synthetic routes, etc., and achieve reduction The effect of simple and controllable reaction and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
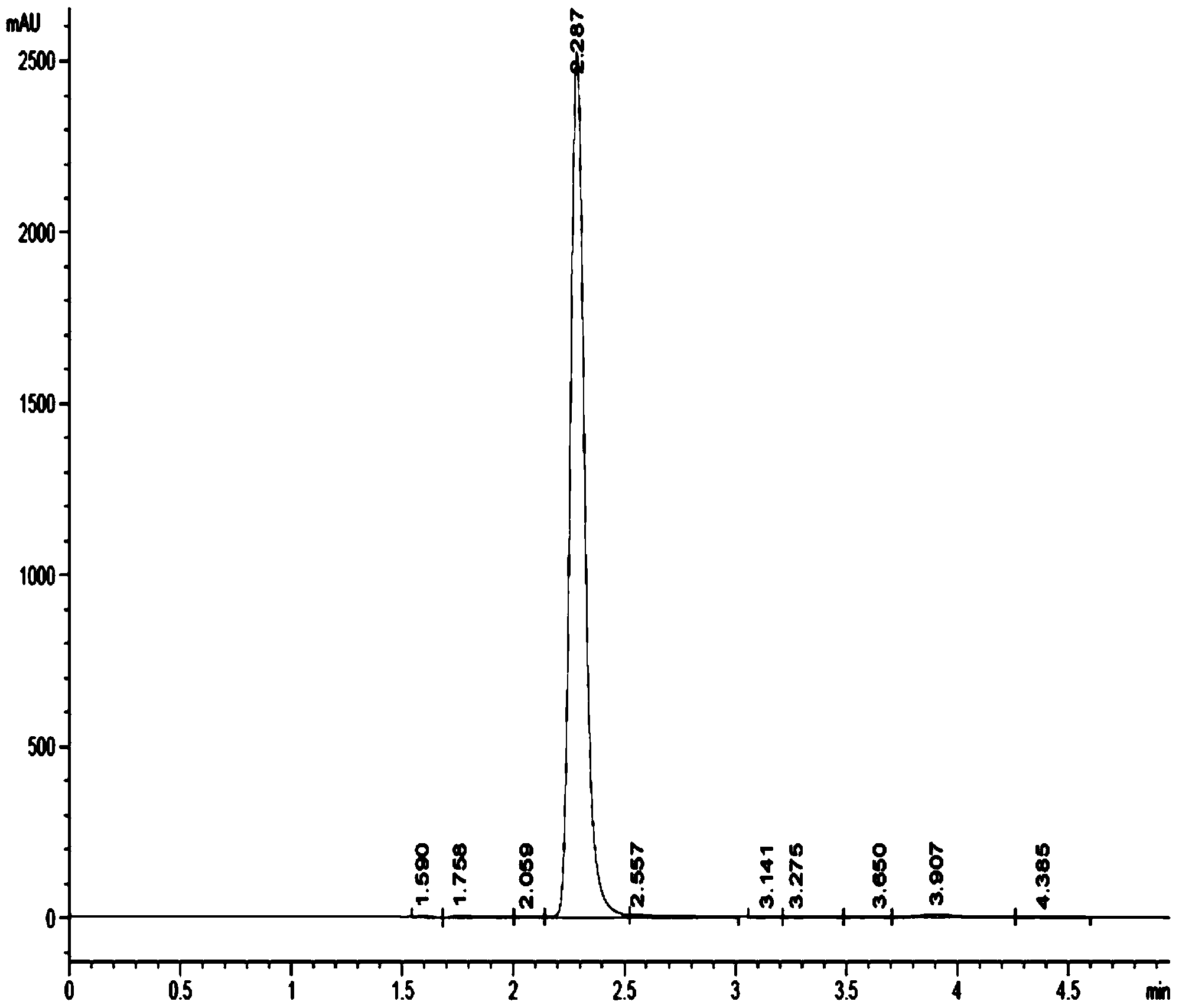
[0030] In a 500ml four-neck flask equipped with an electric stirrer, a thermometer, a reflux condenser and a dropping funnel, add 40 g of o-nitrotoluene, 10 g of ethanol (technical grade), dichloroethane (technical grade) with a concentration of 98%. ) 120g and Raney nickel catalyst 5g, stirred and cooled to 0°C, under this condition, 35g of hydrazine hydrate with a concentration of 80% was added dropwise. The solid catalyst was left standing to separate the water layer, ethanol and dichloroethane were removed by vacuum rotary evaporation, and an orange-red oil was obtained, which was recrystallized with petroleum ether, and after suction filtration and drying, a pure white crystalline product o-formazan was obtained 33.5 g of phenylhydroxylamine, with a liquid chromatography analysis content of 96.4%, and a yield of 91.8%.
Embodiment 2
[0032] In a 500ml four-necked flask equipped with electric stirrer, thermometer, reflux condenser and dropping funnel, adding concentration is 98% o-nitrotoluene 40g, tetrahydrofuran (technical grade) 100g and Raney nickel catalyst 6g, stir Cool to 5°C, add dropwise 35g of hydrazine hydrate with a concentration of 80% under this condition, after the dropwise addition, keep the reaction at 6°C for 6 hours, remove the solid catalyst by suction filtration after cooling, let stand to separate the water layer, The tetrahydrofuran was removed by vacuum rotary evaporation to obtain an orange-red oily substance, which was recrystallized with petroleum ether. After suction filtration and drying, 32.5 g of a pure white crystalline product o-methylphenylhydroxylamine was obtained, and the content was 96.2% according to liquid chromatography analysis. , and the yield was 88.8%.
Embodiment 3
[0034] In a 500ml four-necked flask equipped with electric stirrer, thermometer, reflux condenser and dropping funnel, adding concentration is 98% o-nitrotoluene 40g, tetrahydrofuran (technical grade) 100g and Raney nickel catalyst 6g, stir Cool to 8°C, add dropwise 35g of hydrazine hydrate with a concentration of 80% under this condition, after the dropwise addition, keep the reaction at 10°C for 4 hours, remove the solid catalyst by suction filtration after cooling, let stand to separate the water layer, The tetrahydrofuran was removed by vacuum rotary evaporation to obtain an orange-red oily substance, which was recrystallized with petroleum ether. After suction filtration and drying, 31.5 g of a pure white crystalline product o-methylphenylhydroxylamine was obtained, and the content was 95.6% by liquid chromatography analysis. , the yield was 85.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com