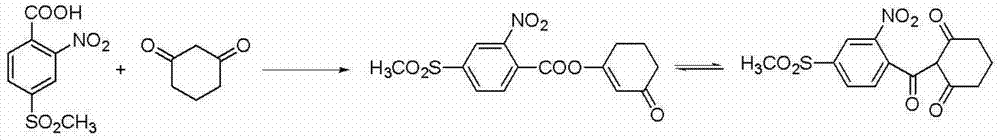
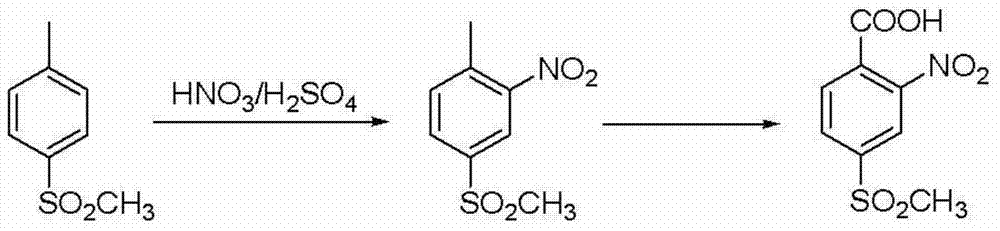
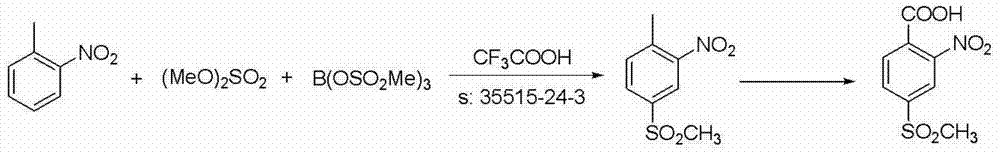
Preparation method of mesotrione
A technology of mesotrione and methylbenzenesulfonyl chloride, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry and other directions, can solve the problems of high cost, lack of competitiveness, expensive anhydrous bromine and the like , to achieve the effect of easy circulation, environmental friendliness, and reduced pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] (1) Synthesis of 3-nitro-4-methylbenzenesulfonyl chloride
[0054] Add chlorosulfonic acid (559.2g / 4.8mol) into a 1L four-neck flask, heat up to 110-115°C, maintain the temperature, slowly add o-nitrotoluene (548.0g / 4.0mol) dropwise, the dropwise addition is complete, Continue the heat preservation reaction for 3.5 hours, stop the reaction, cool the reaction solution to 60-65°C, add 1mL of DMF, maintain the temperature, slowly add thionyl chloride (547.5g / 4.6mol) dropwise, and continue the heat preservation reaction for 3 hours, a mixture of 3-nitro-4-methylbenzenesulfonyl chloride was obtained, which was directly used in the next reaction.
[0055] (2) Synthesis of 2-nitro-4-thiamphenicol toluene (NMST)
[0056] Add sodium sulfite (479.0g / 3.7mol), sodium carbonate (763.0g / 7.2mol) and 6L water in sequence into a 10L four-neck flask, stir and mix well, control the temperature at 0-5°C, and slowly add step (1) The obtained mixed solution of 3-nitro-4-methylbenzenesulfon...
Embodiment 2
[0066] (1) Synthesis of 3-nitro-4-methylbenzenesulfonyl chloride
[0067] Add chlorosulfonic acid (559.2g / 4.8mol) into a 1L four-necked flask, heat up to 90-100°C, maintain the temperature, slowly add o-nitrotoluene (548.0g / 4.0mol) dropwise, the dropwise addition is complete, Continue the heat preservation reaction for 4 hours, stop the reaction, cool the reaction solution to 50-55°C, add 1mL DMF, maintain the temperature, slowly add thionyl chloride (547.5g / 4.6mol) dropwise, after the addition is complete, continue the heat preservation reaction for 4.5 hours , that is, a mixture of 3-nitro-4-methylbenzenesulfonyl chloride was directly used in the next reaction.
[0068] (2) Synthesis of 2-nitro-4-thiamphenicol toluene (NMST)
[0069] In a 10L four-neck flask, add sodium sulfite (479.0g / 3.7mol), sodium carbonate (763.0g / 7.2mol) and 6L water in sequence, stir and mix well, use an ice-water bath to control the temperature at 0-5°C, and slowly drop the steps (1) The obtained m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com