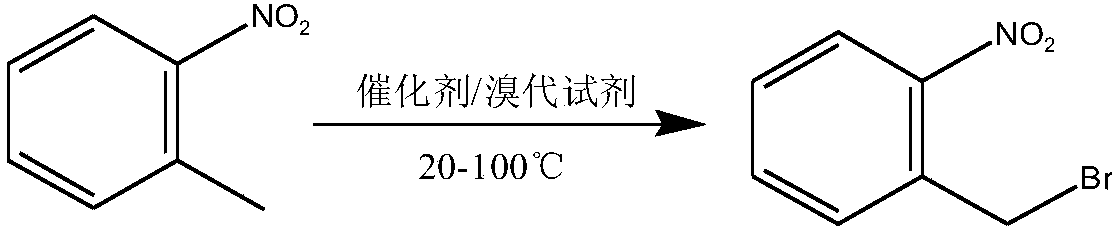
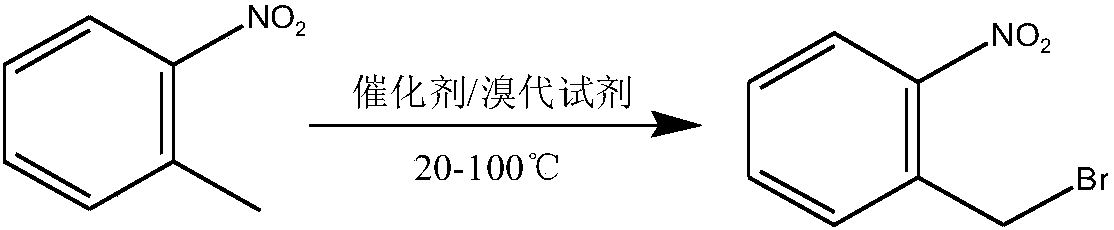
Method used for preparing o-nitrobenzyl bromide
A technology of nitrobenzyl bromide and o-nitrotoluene, which is applied in the field of organic synthesis, can solve the problems of poor mixing effect, generation of acidic waste water, and high operational requirements, and achieves large industrial practical value, high utilization rate, and simple post-treatment. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 127.5 grams of o-nitrotoluene, 325 grams of cyclohexane, 137.2 grams of dibromohydantoin and 6.6 grams of AIBN into a 1L reaction flask equipped with a stirring, thermometer, and condenser tube, and slowly raise the temperature to 80°C, and control the heating rate to prevent Vigorous reflux leads to flushing, reflux for 3-4 hours until the solution is colorless, and the sample is controlled in the center. After passing the test, stop the reaction, cool down to 20 degrees, filter, and the filtrate is precipitated under reduced pressure to 60 degrees to obtain bromide. Normalized to: 90.4% o-nitrobenzyl bromide, 6.5% o-nitrotoluene, 2.2% o-nitrobenzylidene dibromide. The yield of o-nitrobenzyl bromide (in terms of o-nitrotoluene, the same below) reaches 90.1%.
Embodiment 2
[0025] Add 127.5 grams of o-nitrotoluene, 300 grams of 1,2-dichloroethane, 140.2 grams of dibromohydantoin and 6.6 grams of AIBN into a 1L reaction flask equipped with a stirring, thermometer, and condenser, and slowly raise the temperature to 83°C. Control the heating rate to prevent violent reflux from causing flushing, reflux for 3-4 hours until the solution is colorless, take a sample for control, stop the reaction after passing the test, cool down to 20 degrees, filter, and desolvate the filtrate to 60 degrees under reduced pressure to obtain bromide. Normalized to: 91.8% o-nitrobenzyl bromide, 5.3% o-nitrotoluene, 2.1% o-nitrobenzylidene dibromide. The yield of o-nitrobenzyl bromide is 91.1%.
Embodiment 3
[0027] Add 383 grams of o-nitrotoluene, 975 grams of chlorobenzene, 420 grams of dibromohydantoin and 19 grams of AIBN into a 2L reaction flask equipped with a stirring, thermometer, and condenser tube, and slowly raise the temperature to 80°C, and control the heating rate to prevent Punch the material, keep it warm for 4 hours until the red color of bromine fades, take a sample and control it, stop the reaction after passing the test, cool down to 20 degrees, filter, and decompress the filtrate to remove the solvent to obtain the bromide. Normalized to: 89.5% o-nitrobenzyl bromide, 7.3% o-nitrotoluene, 1.9% o-nitrobenzylidene dibromide. The yield of o-nitrobenzyl bromide is 89.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com