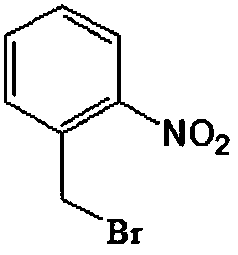
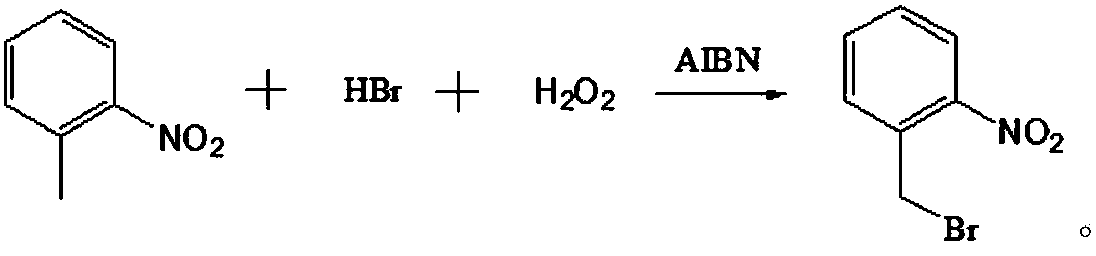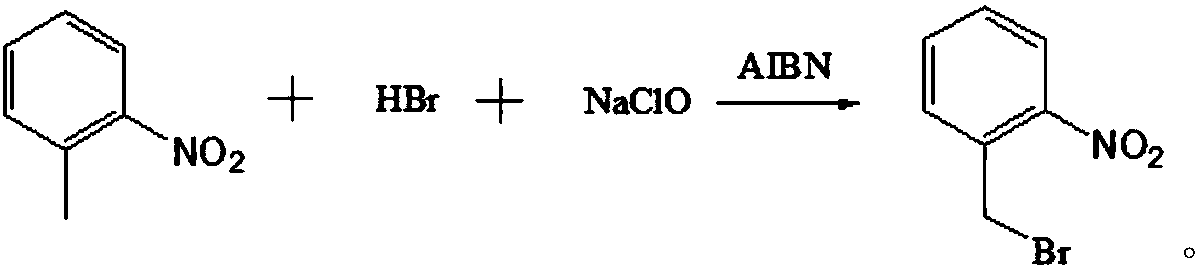Preparation method of o-nitrobenzyl bromide
A technology of o-nitrobenzyl bromide and o-nitrotoluene, which is applied in the field of preparation of o-nitrobenzyl bromide, can solve the problems of low yield, high cost, and expensive raw materials, and achieve high reaction selectivity and low cost. Inexpensive and readily available raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A preparation method of o-nitrobenzyl bromide, using o-nitrotoluene as a raw material, using NaClO / HBr as a bromination agent, and using azobisisobutyronitrile as an initiator to prepare by bromination reaction Ortho-nitrobenzyl bromide, comprising the following steps:
[0030] (1) Prepare the first mixed solution and the second mixed solution:
[0031] 206g dichloroethane is divided into two parts, respectively the first solvent 136g, the second solvent 70g; 2.5g azobisisobutyronitrile (AIBN) is divided into two parts, respectively the first initiator 0.5g, the second initiator Dose 2.0g.
[0032] With 41.2g (0.30mol) o-nitrotoluene, 136g dichloroethane (the first solvent), 54.6g hydrobromic acid solution (the mass concentration of hydrobromic acid in this solution is 40%, promptly contains 0.27mol in this solution hydrobromic acid) and 0.5g azobisisobutyronitrile (the first initiator) were mixed uniformly to obtain the first mixed solution.
[0033] Mix 2.0 g of az...
Embodiment 2
[0037] A preparation method of o-nitrobenzyl bromide, using o-nitrotoluene as a raw material, using NaClO / HBr as a bromination agent, and using azobisisobutyronitrile as an initiator to prepare by bromination reaction Ortho-nitrobenzyl bromide, comprising the following steps:
[0038] (1) Prepare the first mixed solution and the second mixed solution:
[0039] 247g dichloroethane is divided into two parts, respectively the first solvent 177g, the second solvent 70g; 3.5g azobisisobutyronitrile (AIBN) is divided into two parts, respectively the first initiator 0.5g, the second initiator Dose 3.0g.
[0040] With 41.2g (0.30mol) o-nitrotoluene, 177g dichloroethane (the first solvent), 50.6g hydrobromic acid solution (the mass concentration of hydrobromic acid in this solution is 48%, promptly contains 0.30mol hydrobromic acid) and 0.5g azobisisobutyronitrile (the first initiator) were mixed uniformly to obtain the first mixed solution.
[0041] 3.0 g of azobisisobutyronitrile ...
Embodiment 3
[0045] A preparation method of o-nitrobenzyl bromide, using o-nitrotoluene as a raw material, using NaClO / HBr as a bromination agent, and using azobisisobutyronitrile as an initiator to prepare by bromination reaction Ortho-nitrobenzyl bromide, comprising the following steps:
[0046] (1) Prepare the first mixed solution and the second mixed solution:
[0047] 230g carbon tetrachloride is divided into two parts, respectively the first solvent 160g, the second solvent 70g; 2.5g azobisisobutyronitrile (AIBN) is divided into two parts, respectively the first initiator 0.5g, the second initiator Dose 2.0g.
[0048] With 41.2g (0.30mol) o-nitrotoluene, 160g carbon tetrachloride (the first solvent), 66.7g hydrobromic acid solution (the mass concentration of hydrobromic acid in this solution is 40%, promptly contains 0.33mol in this solution hydrobromic acid) and 0.5g azobisisobutyronitrile (the first initiator) were mixed uniformly to obtain the first mixed solution.
[0049] 2.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com