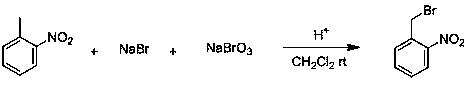Device for continuous preparation of o-nitrobenzyl bromide and preparation method thereof
A technology of nitrobenzyl bromide and o-nitrotoluene, which is applied in the field of continuous preparation of o-nitrobenzyl bromide, can solve the problems of difficult separation and extraction, long reaction time, low conversion rate, etc. The effect of shortening the time and reducing by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
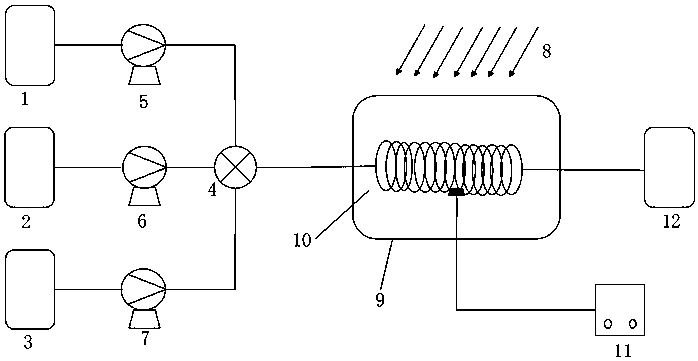
[0030] The structure of the reaction device is as figure 1 As shown, the tubular reactor 10 used in this embodiment has a length of 10 m and a diameter of 1 mm, and the material of the tubular reactor 10 is polytetrafluoroethylene; the light source 8 is a 250 nm UV lamp.
[0031] First turn on the light source 8, turn on the circulating constant temperature water bath glass jacket 9 to preheat the tubular reactor 10 to 20°C, and dissolve o-nitrotoluene in methylene chloride to obtain a 0.28mol / L o-nitrotoluene solution and liquid bromine Dissolve in dichloromethane to obtain 0.28mol / L liquid bromine solution, then store o-nitrotoluene solution, liquid bromine solution and water in storage tank Ⅰ1, storage tank Ⅱ2 and storage tank Ⅲ3 respectively, and the three materials pass through the metering pump respectively Ⅰ5, metering pump Ⅱ6 and metering pump Ⅲ7 measure, control the molar flow ratio of o-nitrotoluene solution, liquid bromine solution, and water to 1:0.4:3, and transpo...
Embodiment 2
[0033] The structure of the reaction device is as figure 1 , the pipe length is 50m, the diameter is 2mm, the pipe material is polytetrafluoroethylene; the light source is a 250nm UV lamp.
[0034] First turn on the light source, turn on the circulating constant temperature water bath glass jacket preheating pipeline, and the reaction temperature is 20°C. Dissolve o-nitrotoluene in dichloromethane to obtain a 0.43mol / L o-nitrotoluene solution, and dissolve liquid bromine in dichloromethane to obtain a 0.43mol / L liquid bromine solution, then dissolve the o-nitrotoluene solution, liquid bromine solution 1. Water is stored in storage tank Ⅰ, storage tank Ⅱ and storage tank Ⅲ respectively, and the three materials pass through metering pump Ⅰ, metering pump Ⅱ and metering pump Ⅲ respectively, and are sent to the mixer at the same time, and the flow rate is controlled so that the molar flow ratio of the three is 1 : 1:4, the material enters the tubular reactor after being mixed by ...
Embodiment 3
[0036] The structure of the reaction device is as figure 1 , the pipe length is 100m, the diameter is 1.5mm, the pipe material is polytetrafluoroethylene; the light source is a 250nm UV lamp.
[0037] Firstly, turn on the light source, turn on the circulating constant temperature water bath glass jacket preheating pipeline, and the reaction temperature is 30°C. Dissolve o-nitrotoluene in dichloromethane to obtain a 0.55mol / L o-nitrotoluene solution, and dissolve liquid bromine in dichloromethane to obtain a 0.55mol / L liquid bromine solution, then dissolve the o-nitrotoluene solution, liquid bromine solution, water Stored in storage tank Ⅰ, storage tank Ⅱ and storage tank Ⅲ respectively, the three materials pass through metering pump Ⅰ, metering pump Ⅱ and metering pump Ⅲ respectively, and are transported to the mixer at the same time, and the flow rate is controlled so that the molar flow ratio of the three is 1:1 : 7, after the material is mixed by a mixer, it enters a tubul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com