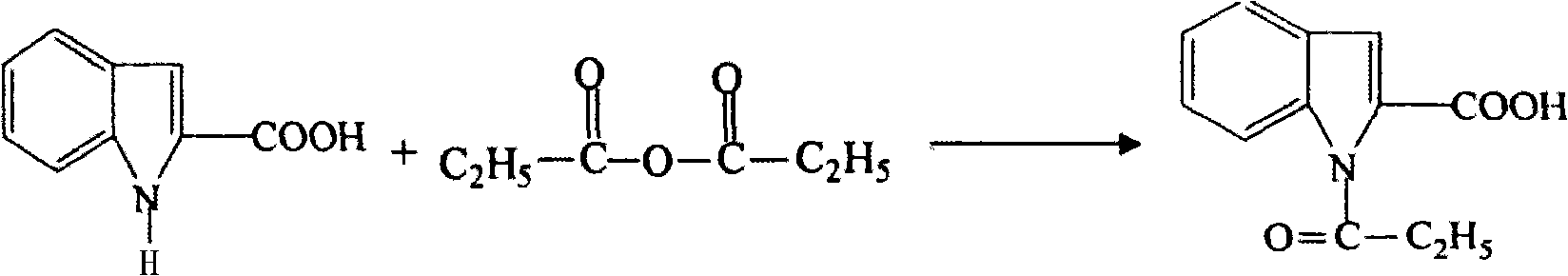Preparation method of L-octohydroindoline-2-formic acid
A technology of indoline and formic acid, which is applied in the field of preparation of L-octahydroindoline-2-carboxylic acid, can solve the problems of high raw material prices and no market advantages, and achieve low cost, environmentally friendly process, and clean hydrogenation route Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
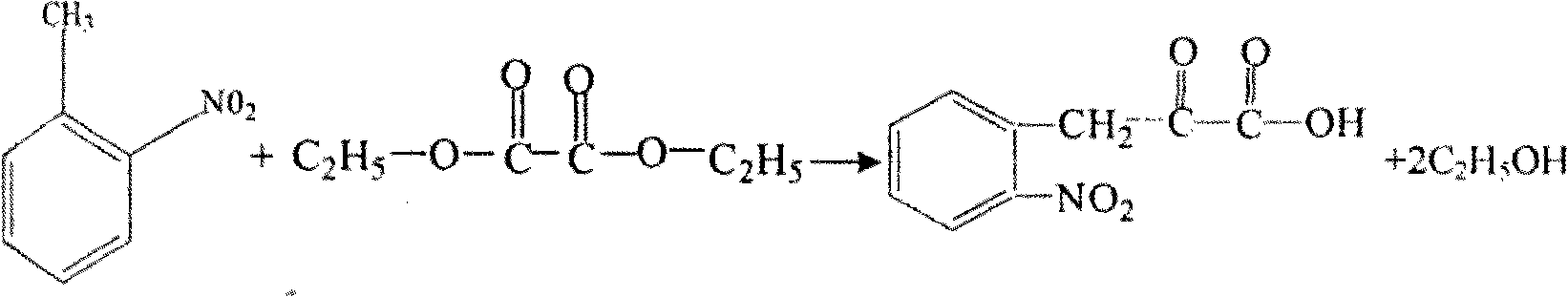
[0006] The preparation method of L-octahydroindoline-2-carboxylic acid:
[0007] (1), the condensation process synthesizes o-nitropyruvate:
[0008] The reaction formula is as follows:
[0009]
[0010] C 2 h 5 Na+HCl→C 2 h 5 OH+NaCl
[0011] Add 185 kilograms of diethyl oxalate, 121 kilograms of o-nitrotoluene and 87 kilograms of sodium ethylate to the reaction kettle, add 2000 l of heat to reflux for 3 hours, evaporate the ethanol, add water to the reaction kettle, stir for deep decomposition, and then steam Remove o-nitrotoluene until the reaction solution is clear, adjust the pH to 0.5 with 30% hydrochloric acid after reaching room temperature, spin dry by centrifugation to obtain a solid, and dry it at 60-70°C.
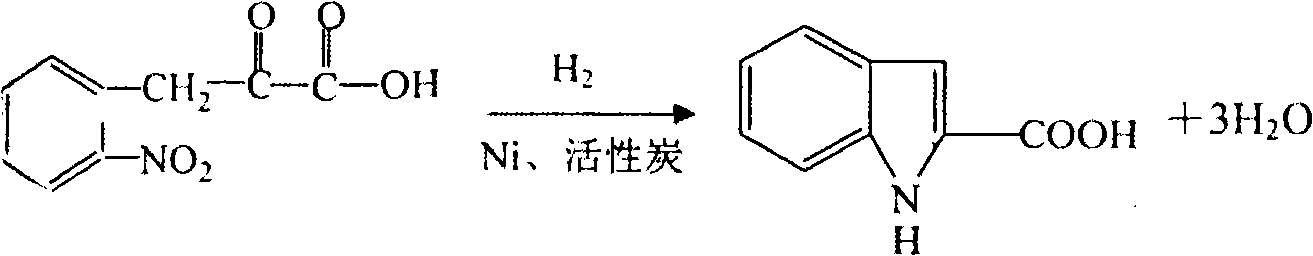
[0012] (2), hydrogenation section prepares indole-2-carboxylic acid:
[0013] The reaction formula is as follows:
[0014]
[0015] Add 81kg of o-nitropyruvate and 600l of 95% ethanol to the reaction kettle, adjust the pH to 7-8 with 30% lye, add ni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com