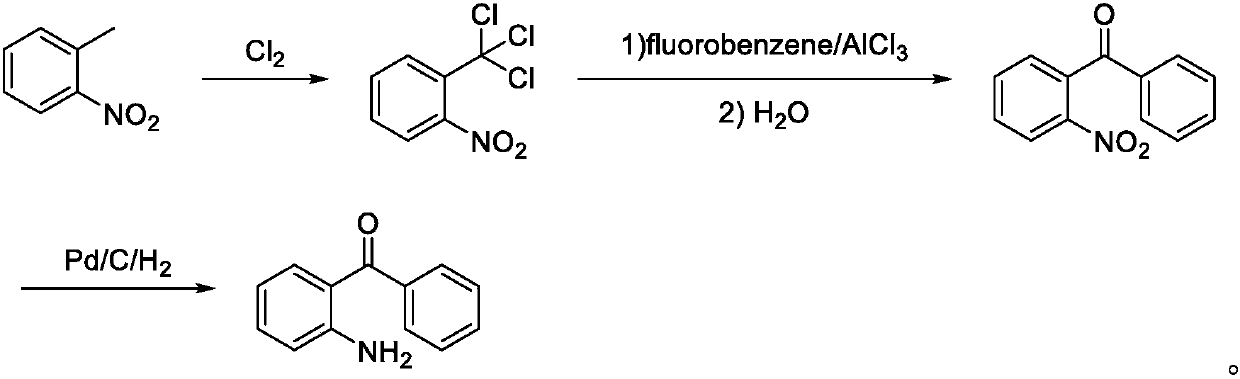
Preparation method of 2-amino-4'-fluoro-diphenyl ketone
A technology of benzophenone and amino group is applied in the field of preparation of 2-amino-4'-fluoro-benzophenone, can solve the problems of difficulty in industrialization, many reagents, complicated operation and the like, and achieves obvious cost advantages and convenient operation. Controllable, easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A preparation method of 2-amino-4'-fluoro-benzophenone, comprising the following steps: o-nitrotoluene is chlorinated by chlorine gas to obtain o-nitrobenzotrichlorotoluene; and then reacted with fluorobenzene by Friedel-Crafts-hydrolysis to obtain 2-nitro-4'-fluoro-benzophenone; and then reduced to 2-amino-4'-fluoro-benzophenone.
Embodiment 2
[0041] A preparation method of 2-amino-4'-fluoro-benzophenone, comprising the following steps: take o-nitrotoluene, heat up to 48°C, pass chlorine gas for 2 hours, heat up to 105°C, continue to pass chlorine gas for 6 hours, cool To room temperature to obtain o-nitrobenzotrichloride, wherein the molar ratio of o-nitrotoluene and chlorine is 1:3.5;
[0042] Mix fluorobenzene and aluminum trichloride evenly, cool down to 10°C, add o-nitrotrichlorotoluene dropwise, keep stirring for 40 minutes after the dropwise addition, then adjust the temperature to room temperature, keep it warm for 5 hours, then cool down to 2°C, add water dropwise , and then heated to 100°C, kept warm for 1.5h, cooled to room temperature to crystallize, filtered, and recrystallized with methanol to obtain 2-nitro-4'-fluoro-benzophenone, wherein the moles of fluorobenzene and aluminum trichloride The ratio is 1:3, and the weight ratio of fluorobenzene and water is 1:4;
[0043] Mix 2-nitro-4'-fluoro-benzoph...
Embodiment 3
[0045] A preparation method of 2-amino-4'-fluoro-benzophenone, comprising the following steps: take o-nitrotoluene, heat up to 42°C, pass chlorine gas for 2 hours, then heat up to 115°C, continue to pass chlorine gas for 4.5 hours, Cool to room temperature to obtain o-nitrobenzotrichloride, wherein the molar ratio of o-nitrotoluene and chlorine is 1:4.5;
[0046] Mix fluorobenzene and aluminum trichloride evenly, cool down to 0°C, add o-nitrotrichlorotoluene dropwise, keep stirring for 60 minutes after the dropwise addition, then adjust the temperature to room temperature, keep it warm for 2 hours, then cool down to 5°C, add water dropwise , then heated to 40°C, kept warm for 4h, cooled to room temperature for crystallization, filtered, and recrystallized with methanol to obtain 2-nitro-4'-fluoro-benzophenone, wherein the molar ratio of fluorobenzene to aluminum trichloride 1:1.2, the weight ratio of fluorobenzene and water is 1:10;
[0047] Mix 2-nitro-4'-fluoro-benzophenone...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com