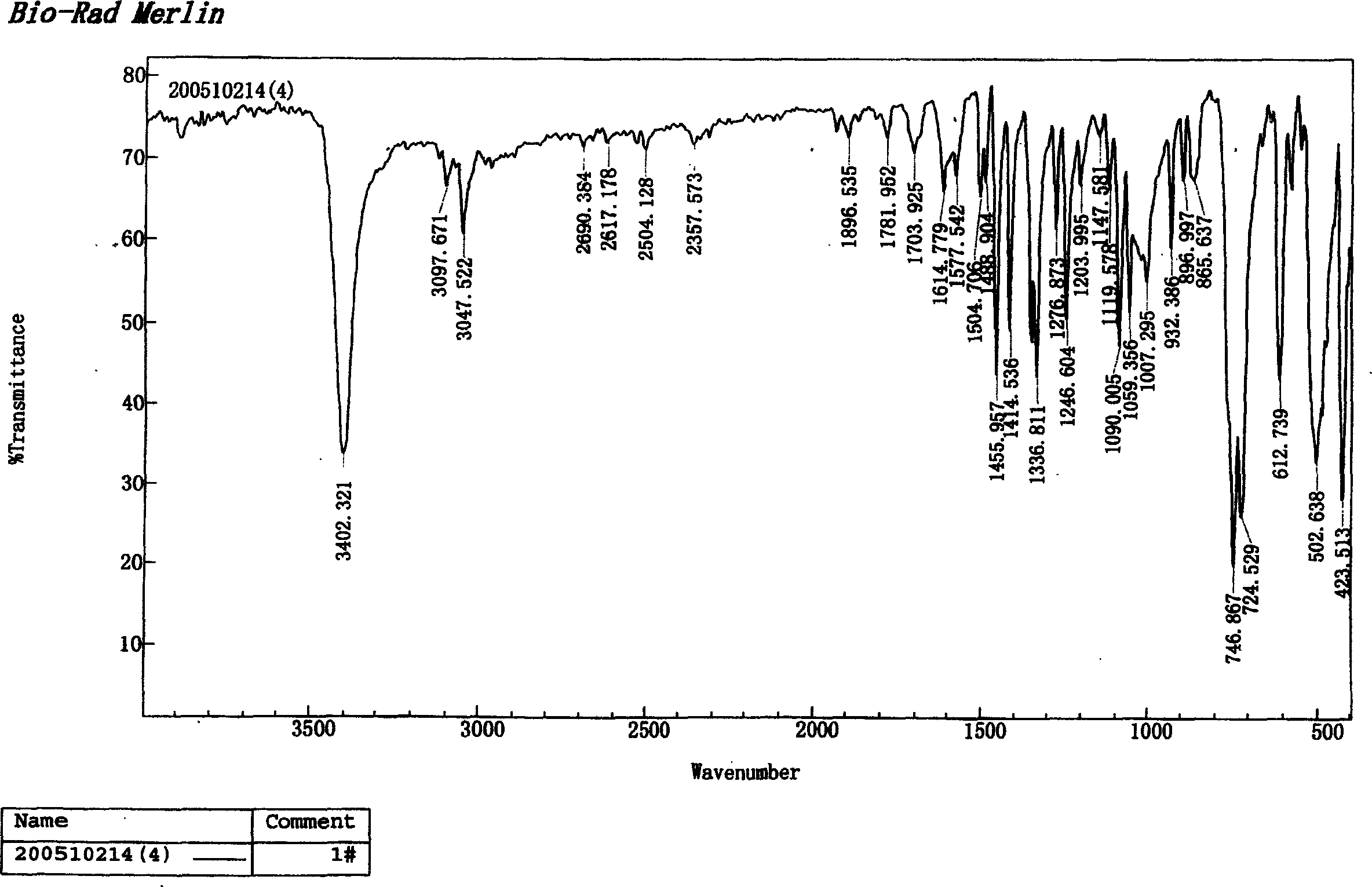
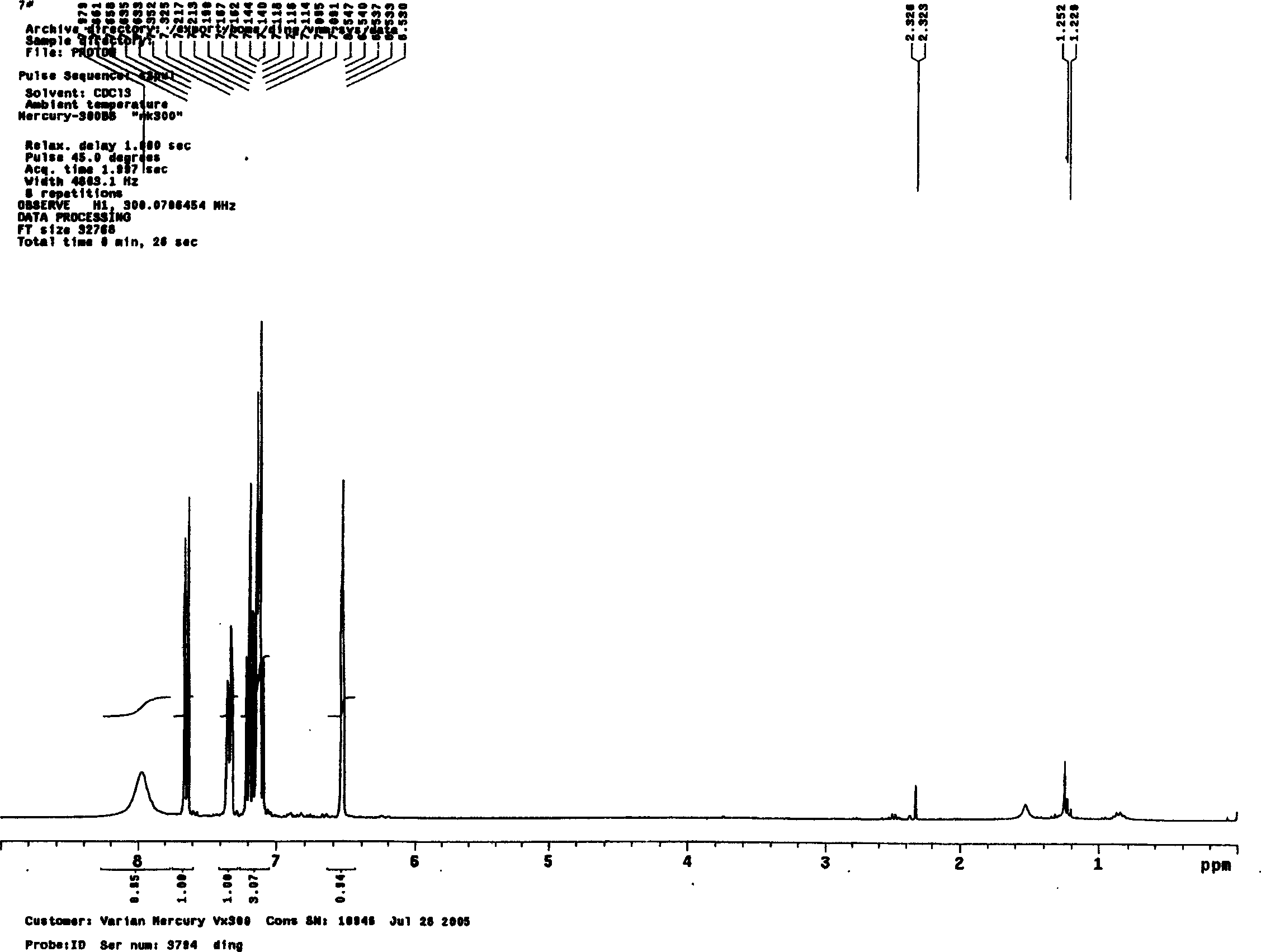
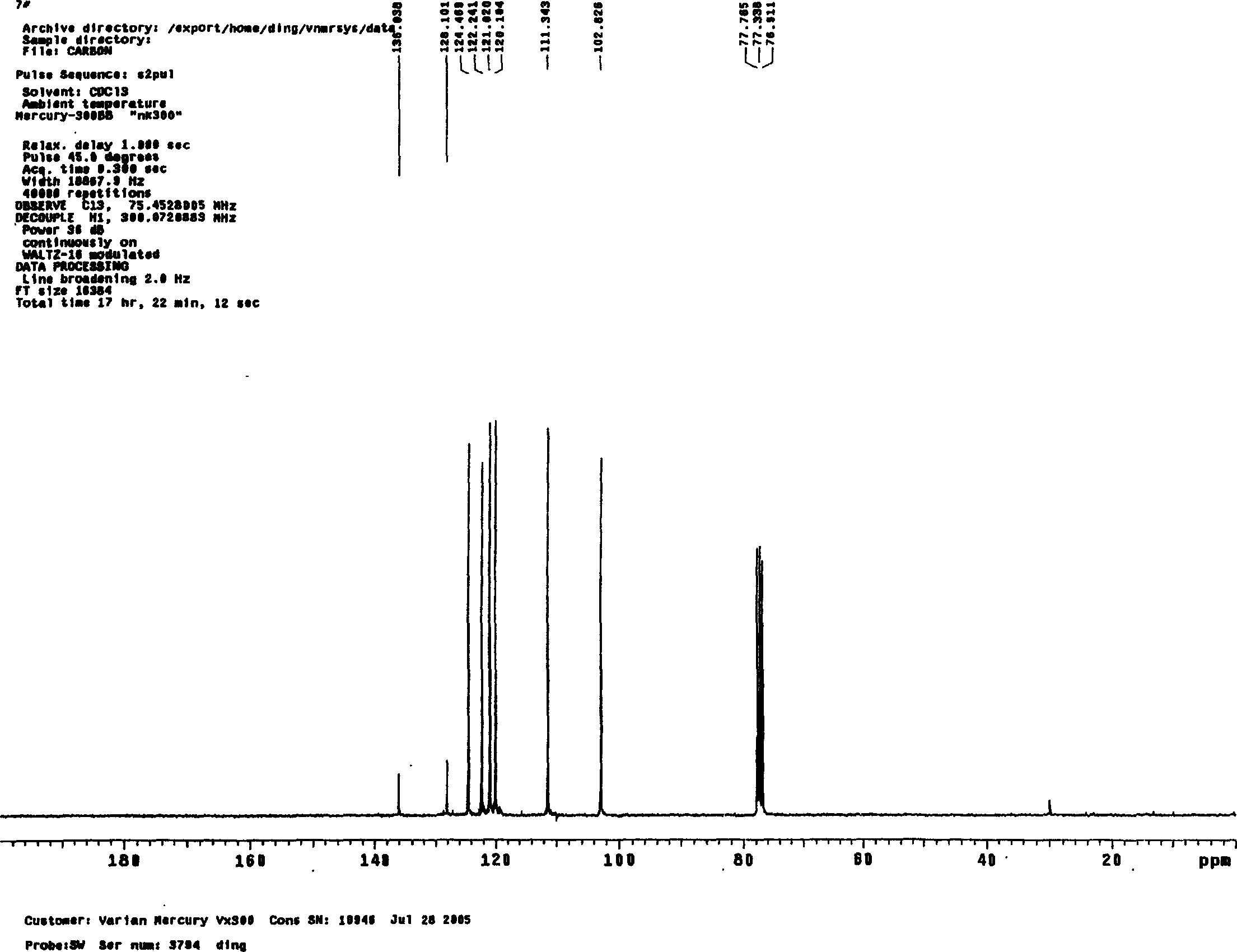
Synthesis method of indole
A synthesis method and technology of indole, applied in the direction of organic chemistry, etc., can solve the problems of three wastes, poor selectivity, long reaction time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A kind of synthetic method of indole is made up of following steps:
[0040] (1) Put 137g o-nitrotoluene, 220g dimethyl sulfoxide, 15g paraformaldehyde, and 5g 20% NaOH aqueous solution into a 1000ml three-necked flask successively. The solution is brownish black at this time. Under stirring, heat up to 50°C for reaction After 70 minutes, the heating was stopped, and concentrated hydrochloric acid was added to the reaction system to adjust the pH to 7. The resulting reaction solution was distilled under reduced pressure, and the previous water-containing fraction was discarded to obtain a mixed solution of DMSO and o-nitrotoluene. The brown-black liquid is the crude product of o-nitrophenylethanol, and the obtained crude product is decompressed and fractionated to remove the dimethylol compound of the by-product o-nitrotoluene to obtain 50 g of o-nitrophenylethanol pure product with a yield of 36.5%. The yield of nitrotoluene is 80%;
[0041] (2) Add 446g of o-nitrop...
Embodiment 2
[0046] Methylolation reaction: Mix potassium hydroxide: o-nitrotoluene: paraformaldehyde: N, N-dimethylformamide in a weight ratio of 1: 1000: 500: 100, stir at 100 ° C, and react After 10 minutes, adjust the pH to 7.5, and conduct fractional distillation under reduced pressure to obtain o-nitrophenylethanol.
Embodiment 3
[0048] Methylolation reaction: Sodium phenoxide in a weight ratio of 1:10:5:50: o-nitrotoluene: 37-41% by weight aqueous formaldehyde solution: Ethylene glycol monomethyl ether is mixed at 150°C Stir and react for 4 hours, adjust the pH to 7, and fractionate under reduced pressure to obtain o-nitrophenylethanol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com