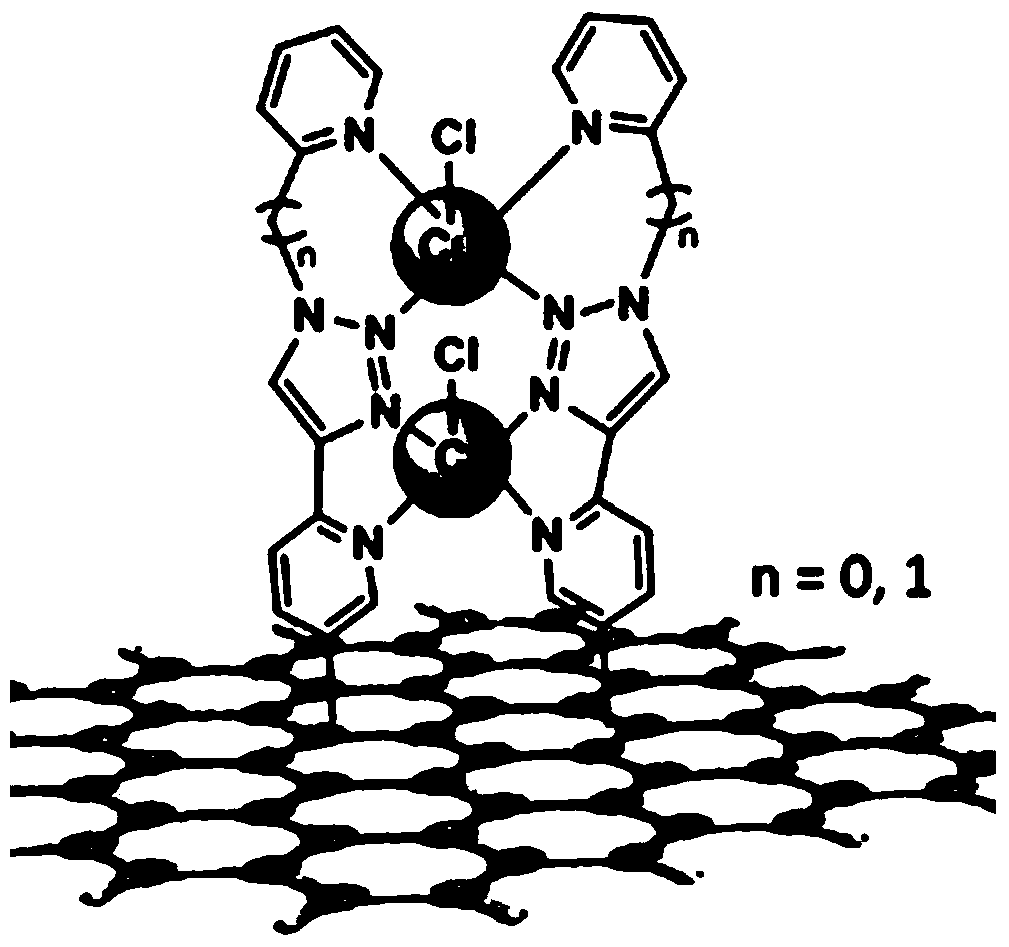
Preparation of Covalently Supported Biomimetic Multinuclear Copper Oxygen Reduction Electrocatalysts on Carbon Materials
An electrocatalyst and multi-core copper technology, applied in the direction of circuits, electrical components, battery electrodes, etc., can solve the problems of poor stability, long-distance targets, and inability to control catalyst reactivity, etc., to increase oxygen flux, improve activity, and catalyst structure. control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Functional modification of triazole groups on the surface of graphene oxide
[0040] Dissolve purified graphene oxide (SP-1graphite, purchased from Bay Carbon Corporation) and triisopropylsilylpyridine in acetonitrile and disperse uniformly by ultrasonic, add a certain amount of isoamyl nitrite solution, heat to 80°C, Under the protection of nitrogen, the reaction was carried out for 24h. The resulting filter cake was filtered, washed with DMF until the filtrate was colorless, and then dispersed in DMF.
[0041] Take a certain amount of the dispersion obtained in the above steps, add tetrabutylammonium fluoride under an ice-water bath, and react for 4 hours at room temperature under a nitrogen atmosphere. Then, a certain amount of copper sulfate pentahydrate, sodium ascorbate and 2-azidopyridine were respectively added therein, and reacted at 50° C. for 36 h under a nitrogen atmosphere. Filter the obtained filter cake, wash with DMF, 50mmol / L EDTA aqueous so...
Embodiment 2
[0042] Example 2 Coordination loading of copper salt on the surface of reduced graphene oxide
[0043] Mix 10.0mg modified reduced graphene oxide with 20.0mg CuCl 2 2H 2 O was dispersed in 20.0 mL of acetonitrile and stirred at room temperature for 24 h. The complex was filtered, washed three times with a small amount of acetonitrile and water, dried in vacuum for 4 h, and the target compound obtained in the above steps was stored in a dark place under a nitrogen atmosphere.
Embodiment 3
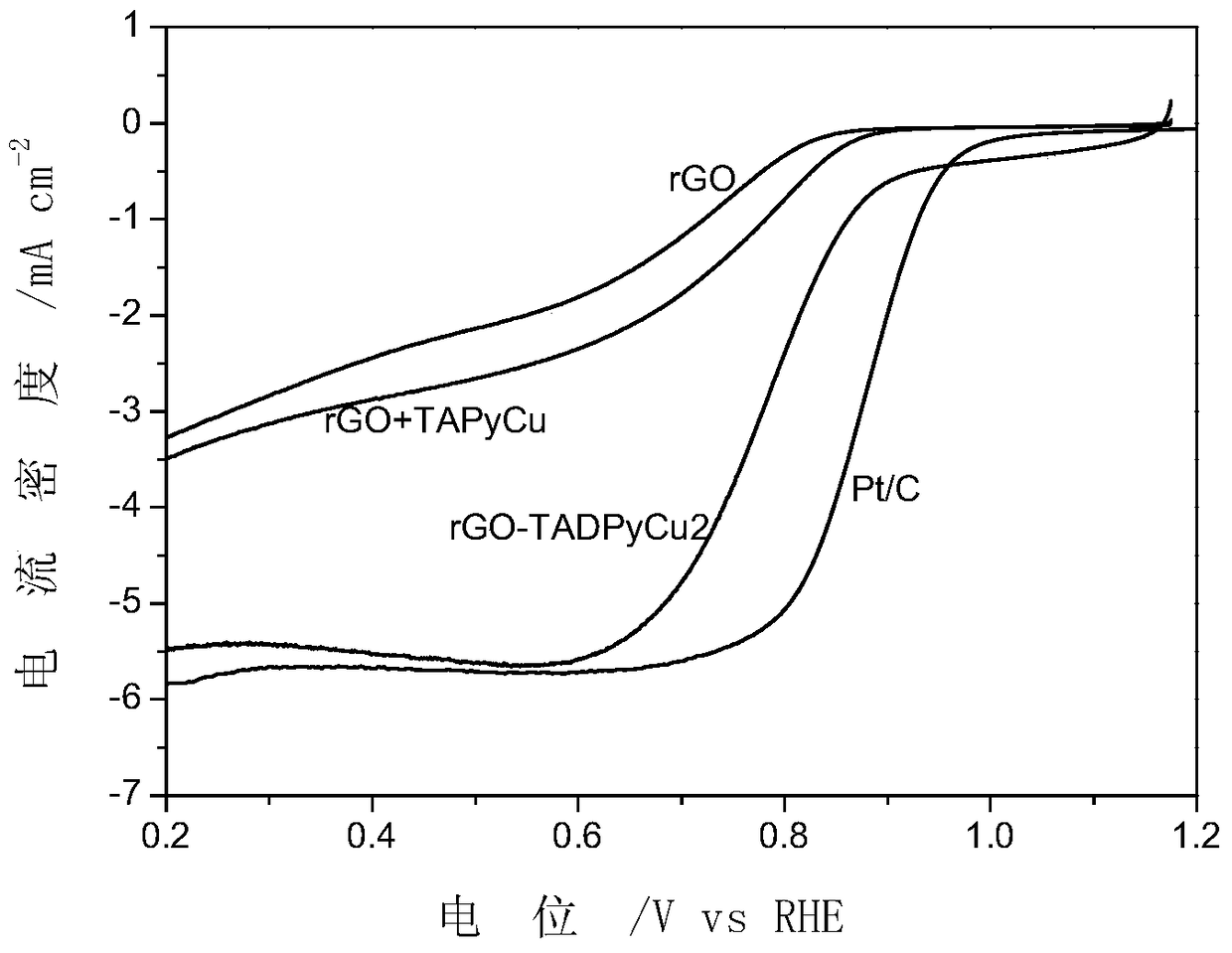
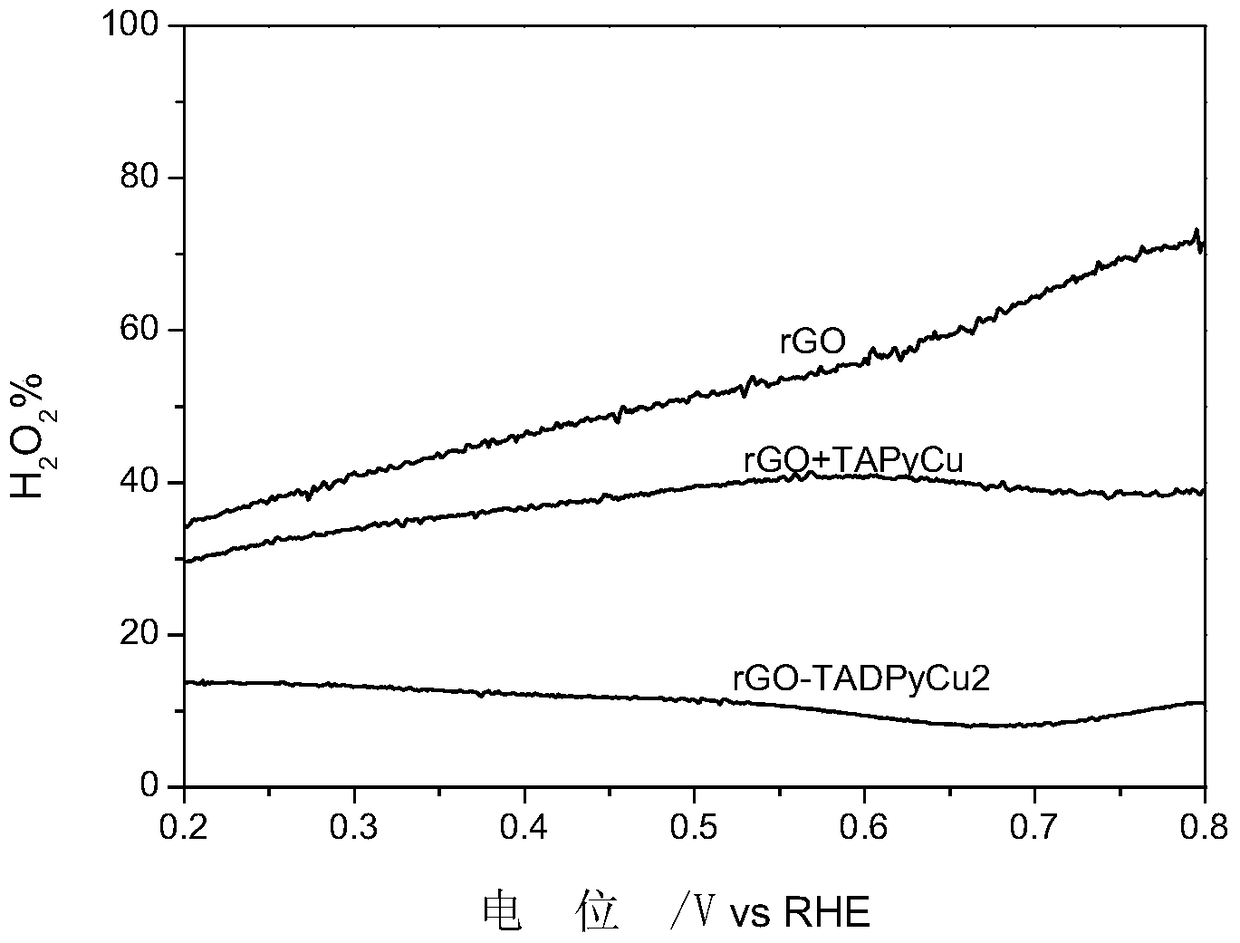
[0044] Example 3 Preparation of rGO+TAPyCu
[0045] Preparation of TAPyCu
[0046] Dissolve a certain amount of 2-azidopyridine and 2-ethynylpyridine in DMF, add a certain amount of copper sulfate pentahydrate and sodium ascorbate, heat to 80°C, and react for 36 hours under nitrogen protection. After treatment, wash with a large amount of water to remove DMF, extract with dichloromethane to obtain an organic phase, and obtain the target product TAPy by column chromatography. A certain amount of TAPy and CuCl 2 2H 2 O was dispersed in acetonitrile, and after stirring at room temperature for 24 h, the post-treatment was washed with acetonitrile for several times until the filtrate was colorless, and the target product TAPyCu was obtained.
[0047] Preparation of rGO+TAPyCu
[0048] Take a certain amount of TAPyCu and rGO and disperse them in acetonitrile, stir at room temperature for 16 hours, filter and dry to obtain the target product rGO+TAPyCu.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com