Cobalt (ii) complexes of quinolinone derivatives and their synthesis methods and applications
A synthesis method and technology of complexes, applied in the field of medicine, can solve problems such as synthesis and application that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
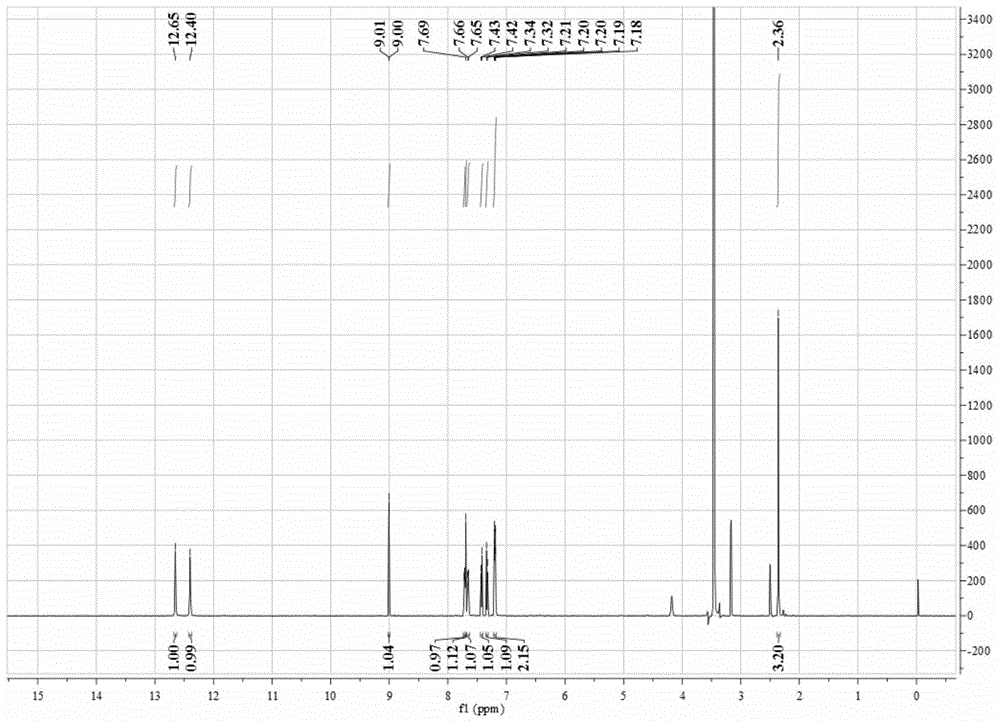
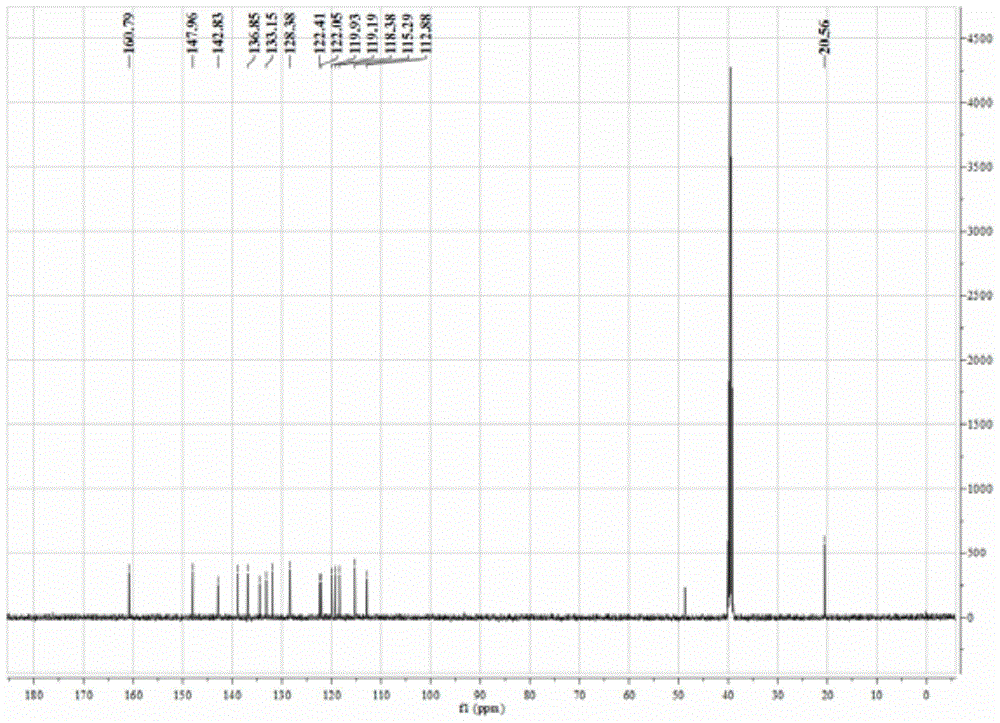
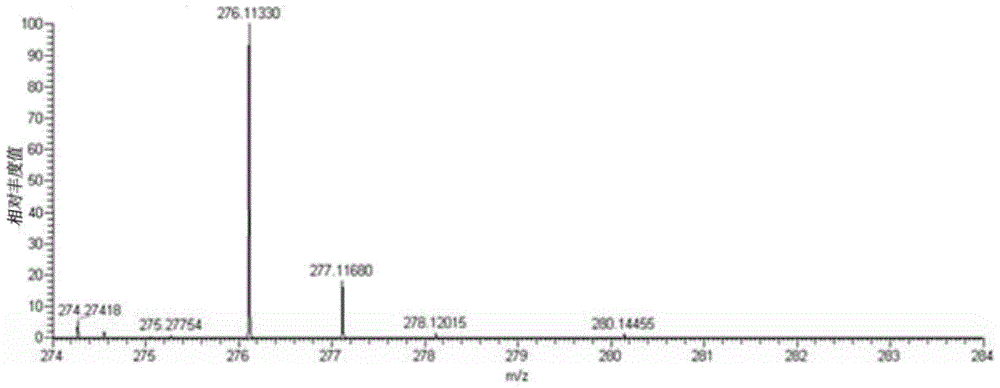
[0060] In a thick-walled borosilicate glass tube open at one end, directly add 0.1 mmol CoCl 2 ·6H 2 O and 0.1 mmol BMQ, and then add 0.6 ml methanol / chloroform mixed solution (the volume ratio of methanol and chloroform is 3:1). Under the condition of vacuuming, the open end was melted and sealed, and then fully reacted at 80° C. for 72 hours to obtain a blue crystalline solid product. The product was subjected to infrared spectroscopy (e.g. Figure 4 shown), elemental analysis, electrospray mass spectrometry (such as Figure 5 shown) combined with X-ray single crystal diffraction analysis for structure (eg Figure 6 Shown) determination, identified as the target complex [Co(BMQ)Cl 2 ], its structural formula is shown in the following formula (I).
[0061]
Embodiment 2
[0063] Repeat Example 1, the difference is:
[0064] 1) The polar solvent was changed to methanol;
[0065] 2) The reaction temperature was changed to 50° C., and the reaction time was changed to 50 h.
[0066] The obtained product was determined to be the target complex [Co(BMQ)Cl 2 ].
Embodiment 3
[0068] 1) Weigh 0.1mmol CoCl respectively 2 ·6H 2 0 and 0.1mmol BMQ, after mixing, be dissolved in 80mL methanol / acetone mixed solution (the volume ratio of methanol and acetone is 1:1), obtain mixed solution;
[0069] 2) The resulting mixed solution was refluxed at 100°C for 8 hours;
[0070] 3) Part of the solvent was evaporated from the obtained reaction solution, and the product was left to stand, and a blue crystalline solid product was precipitated. The solid was separated, washed with ether, and dried.
[0071] The obtained product was determined to be the target complex [Co(BMQ)Cl 2 ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com