A kind of supported lacquer original nickel catalyst and preparation method thereof
A catalyst and lacquer raw nickel technology, applied in the field of integral lacquer raw nickel catalyst and its preparation, can solve the problems of complicated reaction process control, short reaction residence time, etc., and achieve the effects of optimizing the reaction process, improving the catalytic reaction efficiency and uniform distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
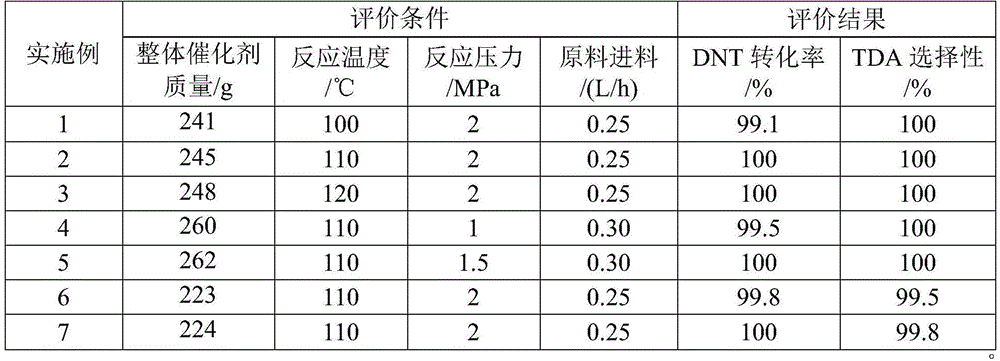
Embodiment 1
[0028] (1) Φ6×10cm, the hole density is 260cpsi, the bulk density is 0.6g / cm 3 The ceramic carrier was immersed in a silica sol with a solid content of 30% at 55°C and stirred continuously. After 2 minutes, it was slowly taken out and purged with air, dried at 110°C for 1 hour, and then baked in a muffle furnace at 300°C for 3 hours to obtain a coating. carrier;
[0029] (2) The coated carrier is put into a vacuum chamber, and then the vacuum chamber is filled with Zn(CH 3 ) 2 , After 1min, vacuumize to 130Pa. After scanning the surface of the carrier with a frequency-doubled ultraviolet continuous argon ion laser (λ=250nm, P=3.000mw) for 4min, fill the vacuum chamber with 7% Zn(CH 3 ) 2 , irradiate with defocused pulsed excimer ArF laser (λ=195nm, 12mJ / pulse, 10ns) for 60s, and load Zn on the carrier coating;
[0030] (3) Configure 300ml of 0.5mol / L nickel chloride solution, immerse the Zn-loaded carrier in step (2) into the prepared solution and react for 1h under stirr...
Embodiment 2
[0033] (1) Φ6×10cm, the hole density is 260cpsi, the bulk density is 0.6g / cm 3 The ceramic carrier was immersed in silica gel with a solid content of 30% at 55°C and stirred continuously. After 3 minutes, it was slowly taken out and purged with air, dried at 110°C for 1 hour, and then baked in a muffle furnace at 500°C for 1 hour to obtain a coated carrier. ;
[0034](2) The coated carrier is put into a vacuum chamber, and then the vacuum chamber is filled with Zn(CH 3 ) 2 , After 3min, vacuumize to 140Pa. After scanning the surface of the carrier with a frequency-doubled ultraviolet continuous argon ion laser (λ=255nm, P=2.500mw) for 6min, fill the vacuum chamber with 9% Zn(CH 3 ) 2 , with a defocused pulsed excimer ArF laser (λ=192nm, 10mJ / pulse, 10ns) irradiated for 70s, Zn is loaded on the carrier coating;
[0035] (3) Configure 300ml of 0.75mol / L nickel chloride solution, immerse the Zn-loaded carrier in step (2) into the prepared solution and react for 1h under stir...
Embodiment 3
[0038] (1) Φ6×10cm, the hole density is 260cpsi, the bulk density is 0.6g / cm 3 The ceramic carrier was immersed in silica gel with a solid content of 30% at 55°C and stirred continuously. After 2 minutes, it was slowly taken out and purged with air, dried at 110°C for 1 hour, and then baked in a muffle furnace at 400°C for 2 hours to obtain a coated carrier. ;
[0039] (2) The coated carrier is put into a vacuum chamber, and then the vacuum chamber is filled with Zn(CH 3 ) 2 , After 1min, vacuumize to 135Pa. After scanning the surface of the carrier with a frequency-doubled ultraviolet continuous argon ion laser (λ=257nm, P=2.000mw) for 7min, fill the vacuum chamber with 12% Zn(CH 3 ) 2 , with a defocused pulsed excimer ArF laser (λ=195nm, 11mJ / pulse, 8ns) irradiated for 70s to load Zn on the carrier coating;
[0040] (3) Configure 300ml of 1mol / L nickel chloride solution, immerse the Zn-loaded carrier in step (2) in the prepared solution and react for 2h under stirring i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com