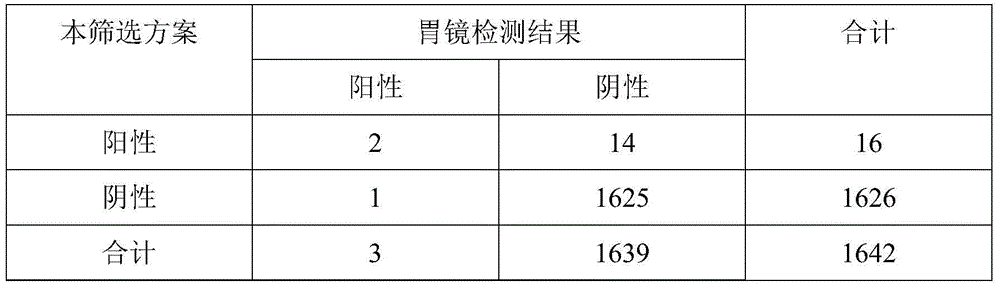
Combined standard quantity for determining quantity of marker used in early gastric cancer risk detection and application thereof in early gastric cancer screening
A technology for early gastric cancer and risk detection, which is applied in the medical field and can solve the problems of low specificity, unsatisfactory specificity and low sensitivity of the screening scheme for hyperglycemia alone.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0026] Perform gastrin-17 (G-17) and pepsinogen I / II (PGI / PG Ⅱ) in the venous blood of the candidates for comparative analysis.
Embodiment example 2
[0028] Gastrin 17 (G-17) pepsinogen I / II (PGI / PG Ⅱ) amount detection method:
[0029] The method of ELISA was adopted to detect, and the detection kit was purchased from Biohit, Finland.
Embodiment example 3
[0031] 1174 cases of gastroscopy in Dingyuan, Anhui (1 case of gastric cancer):
[0032] G-17≥15pmol / L and PGⅠ≤70ng / ml and PGⅠ / PGII≤7; or PG-17≤1pmol / L and PGⅠ≤70ng / ml and PGⅠ / PGⅡ≤7 detected early There were 10 high-risk patients with gastric cancer, and the results of gastroscopy included 1 case of early gastric cancer. The remaining 9 patients all had chronic atrophic gastritis (5 cases in the active stage), including 3 cases with intestinal metaplasia, 2 cases with glandular low-grade intraepithelial neoplasia, and 2 cases with gastric polyps.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com