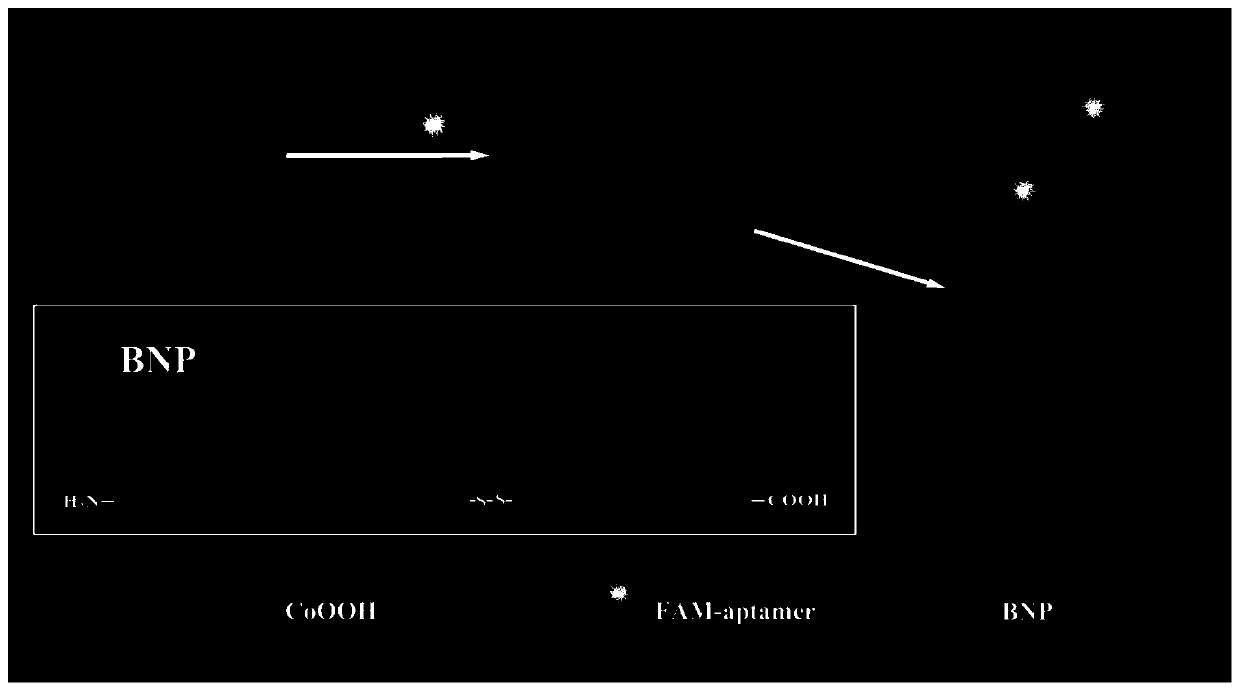
Method for detecting brain natriuretic peptide through composite probe fluorescence "turning on-turning off-turning on" strategy on basis of cobalt nanomaterial/nucleic acid aptamer
A nucleic acid aptamer and composite probe technology, applied in the field of fluorescence analysis, can solve the problems of cumbersome operation, high cost, and time-consuming, and achieve the effect of good selectivity and strong practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
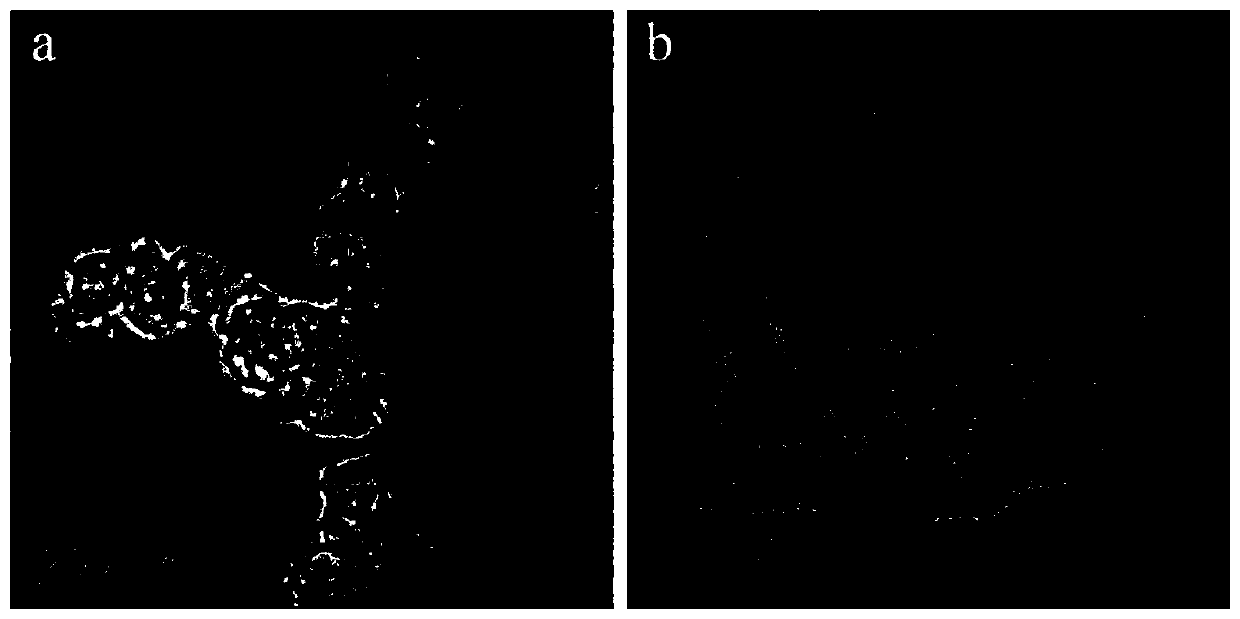
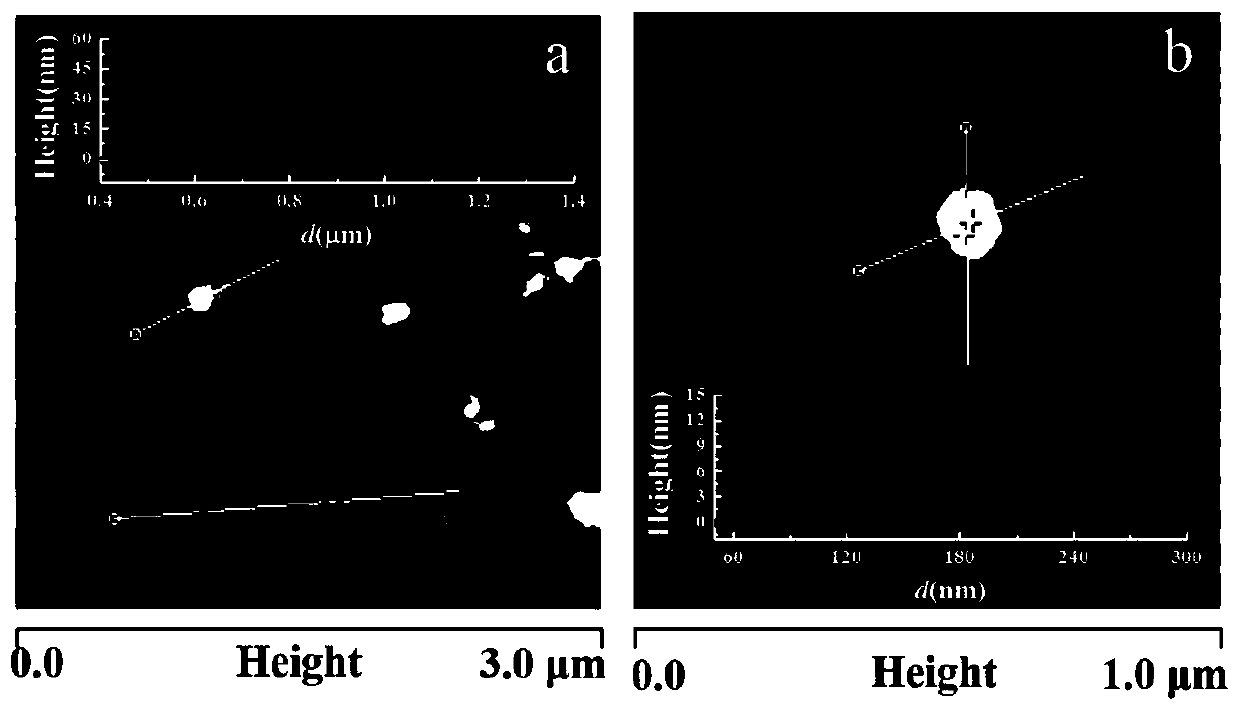
[0044] Preparation and characterization of embodiment 1 CoOOH nanomaterial
[0045] The preparation method of CoOOH nanometer material comprises the following steps:
[0046] (1) 10mL NaOH solution (1mol / L) was quickly added to 40mL CoCl under magnetic stirring 2 ·6H 2O (10mmol / L) solution, after stirring and mixing, sonicate for 1min; (2) add 2mL of NaClO solution with a concentration of 0.9mol / L dropwise during sonication, and continue sonicating for 10min; (3) gradually add to the mixed solution Add 1mol / L HCl dropwise to adjust the pH of the solution to 7.3; (4) Transfer the above mixed solution to a centrifuge tube, centrifuge at 10,000rpm for 10min, discard the supernatant, and ultrasonically disperse the lower precipitate in 25mL ultrapure water, Repeat the centrifugal washing operation at least 3 times; (5) place the last precipitate in an oven, and dry it at 60° C. to obtain the final product (brown black powder), which is ready for use.
[0047] Characterization o...
Embodiment 2
[0052] The preparation and detection method of embodiment 2 solution
[0053] Preparation of FAM-aptamer stock solution
[0054] The freeze-dried powder of FAM-aptamer was centrifuged at 6000 rpm for 5 min, and 800 μL of ultrapure water was added to prepare a stock solution of 2 μmol / L, which was stored in a refrigerator at 4°C. Dilute the concentration to 100nmol / L before use.
[0055] Preparation of BNP standard stock solution
[0056] Add 2mL of ultrapure water to the BNP freeze-dried powder, and equilibrate at room temperature (20-30°C) for 15-20min to completely dissolve the freeze-dried powder, then gently rotate / invert the reagent until uniform, and the concentration of the prepared stock solution is 1273pg / mL, stored in -20°C refrigerator, diluted before use.
[0057] Preparation of CoOOH Nanoring Dispersion
[0058] Weigh 0.0063 g of CoOOH nanorings, add 250 mL of ultrapure water, and sonicate until the CoOOH nanomaterials are evenly dispersed.
[0059] Detection...
Embodiment 3
[0064] Embodiment 3 influence factor investigation
[0065] The inventors studied the influence of the concentration of CoOOH nanorings and the pH of the system on the detection results, as follows.
[0066] Effect of CoOOH nanoring concentration: Add different concentrations of CoOOH nanoring dispersions to the mixed solution of Tris-HCl buffer (pH 7.4) and FAM-aptamer (3.75nmol / L) in turn, and dilute to 400 μL, vortex mixed, let stand at room temperature for 30 min, and measure according to the fluorescence parameters in Example 2.
[0067] When the CoOOH nanoring concentration is 0-18.75 μg / mL, the fluorescence emission intensity of FAM-aptamer is as follows Figure 4 shown. It can be seen that with the increase of the concentration of CoOOH nanorings, the fluorescence intensity of FAM-aptamer gradually decreases. When the concentration of CoOOH nanorings is 18.75 μg / mL, the fluorescence quenching efficiency reaches 80%. conditions for subsequent experiments.
[0068] I...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com