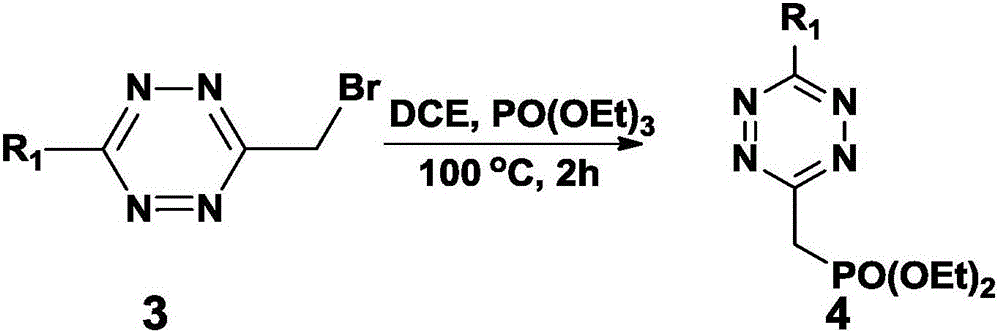Tetrazine compound, method for preparing same and application of tetrazine compound
A compound, tetrazine technology, applied in the field of preparation of tetrazine compounds, can solve problems such as the limited structure of tetrazine derivatives, the application of tetrazine bio-orthogonal fluorescent probes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] In this example, R 1 is a methyl group, and R is a methyl group.
[0065] The preparation method of tetrazine compound comprises the following steps:
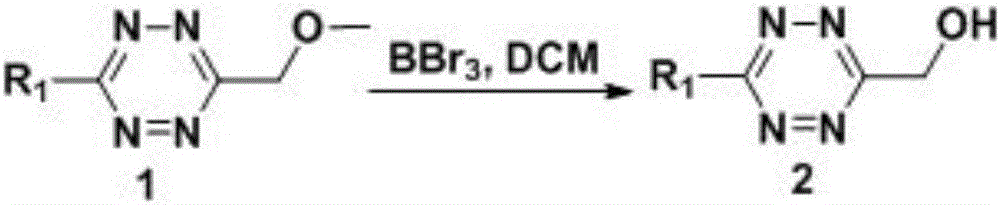
[0066] 1), in a 50mL round-bottomed flask, dissolve compound 1 (280mg, 2mmol) in dry dichloromethane (1mL), slowly add a solution of boron tribromide in dichloromethane (0.4M, 2mL) dropwise to react In the bottle, the reaction solution was in N 2 After reacting at -78–25°C for 3 h under protection, the reaction solution was poured into water, shaken to separate the liquid, washed with water, and the solvent was removed by rotary evaporation to obtain compound 2 (219 mg), yield: 87%;
[0067] The structural formula of compound 1 is: The structural formula of compound 2 is:
[0068] Wherein, the preparation process of compound 1 is:
[0069] In a 50 mL round bottom flask, CH 3 CN (acetonitrile) (410mg, 10mmol) and methoxyacetonitrile (213mg, 3mmol) were dissolved in dry dioxane (1mL), zinc trifluoromethanesulfonat...
Embodiment 2
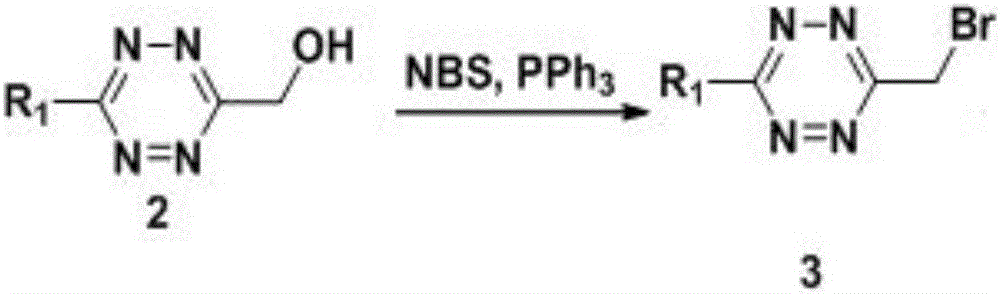
[0079] This example is based on Example 1, and the preparation methods of Compound 1-Compound 4 are consistent.
[0080] Compared with embodiment 1: have following difference: used alkali is tBuOK.
[0081] Compound 4 prepared in this example is used to prepare tetrazine derivatives 1:
[0082] in N 2 Under protection, 0.2mmol of compound 4 was added to 0.16mmol of acetaldehyde in THF solution, 0.22mmol of tBuOK was added, and reacted at 25°C for 2h. Ether:ethyl acetate=2:1 The product was separated and purified, and then dried to obtain tetrazine derivative 1, yield: 35%.
Embodiment 3
[0084] This example is based on Example 1, and the preparation methods of Compound 1-Compound 4 are consistent.
[0085] Compared with embodiment 1: have following difference: used alkali is Cs 2 CO 3 .
[0086] Compound 4 prepared in this example is used to prepare tetrazine derivatives 1:
[0087] in N 2 Under protection, add 0.2mmol of compound 4 to 0.16mmol of acetaldehyde in THF solution, add 0.22mmol of Cs2 CO 3 , and reacted at 25° C. for 2 h. After the reaction, the excess solvent was removed in vacuum, and then the product was separated and purified by thin-layer chromatography (petroleum ether: ethyl acetate=2:1, and then dried to obtain tetrazine derivative 1 , Yield: 25%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com