A combined standard quantity for judging the amount of markers for risk detection of early gastric cancer and its application in screening early gastric cancer
A technology for early gastric cancer and risk detection, applied in the medical field, can solve the problems of unsatisfactory screening program specificity, low sensitivity, and low specificity of high G-17 hyperemia screening program alone.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0026] Perform gastrin-17 (G-17) and pepsinogen I / II (PGI / PG Ⅱ) in the venous blood of the candidates for comparative analysis.
Embodiment example 2
[0028] Gastrin 17 (G-17) pepsinogen I / II (PGI / PG Ⅱ) amount detection method:
[0029] The method of ELISA was adopted to detect, and the detection kit was purchased from Biohit, Finland.
Embodiment example 3
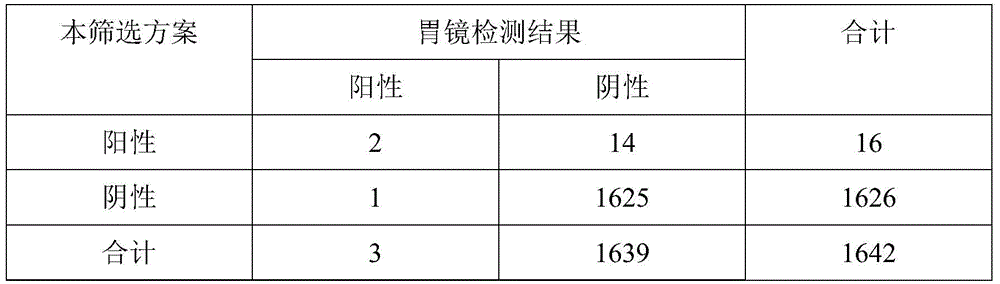
[0031] 1174 cases of gastroscopy in Dingyuan, Anhui (1 case of gastric cancer):
[0032] G-17≥15pmol / L and PGⅠ≤70ng / ml and PGⅠ / PGII≤7; or PG-17≤1pmol / L and PGⅠ≤70ng / ml and PGⅠ / PGⅡ≤7 detected early There were 10 high-risk patients with gastric cancer, and the results of gastroscopy included 1 case of early gastric cancer. The remaining 9 patients all had chronic atrophic gastritis (5 cases in the active stage), including 3 cases with intestinal metaplasia, 2 cases with glandular low-grade intraepithelial neoplasia, and 2 cases with gastric polyps.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com