A kind of fluorophenyl ruthenium compound and its preparation method and application
A kind of fluorobenzene ruthenium compound and ruthenium compound technology are applied in the field of ruthenium compound preparation, and achieve the effects of strong inhibitory activity, low cost and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
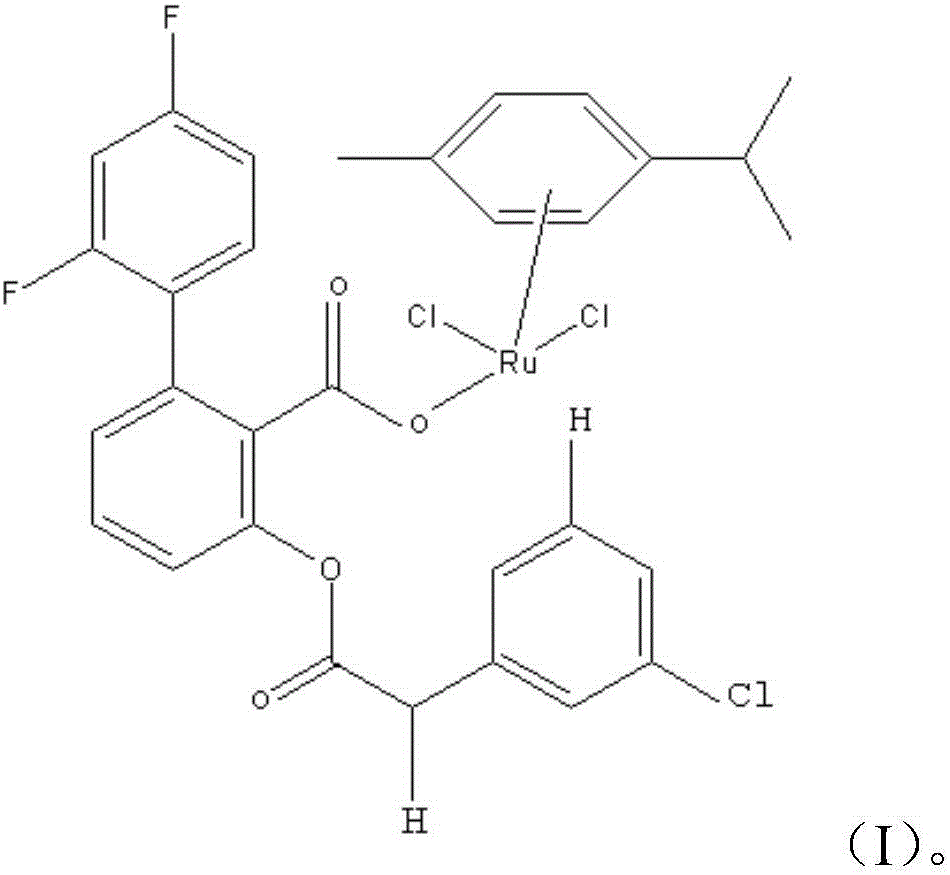
[0026] A fluorophenyl ruthenium compound, said fluorophenyl ruthenium compound has the structure of (I):
[0027]
[0028] A preparation method of fluorophenyl ruthenium compound, comprising the following steps:
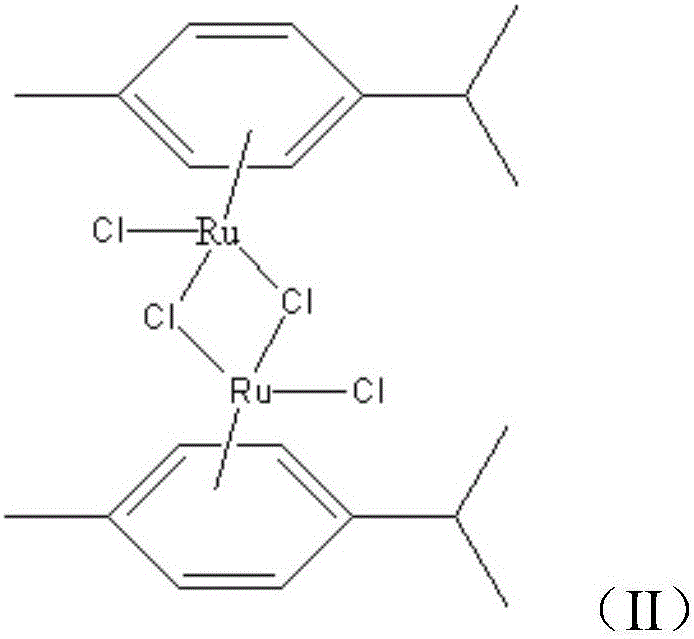
[0029] Step 1. Dissolve 1g of ruthenium trichloride hydrate and 30mL of γ-terpinene in 70mL of absolute ethanol, heat and reflux and stir for 6 hours, and leave to precipitate to obtain dichloro-dichloro-di-methylisopropyl Benzodiruthenium;
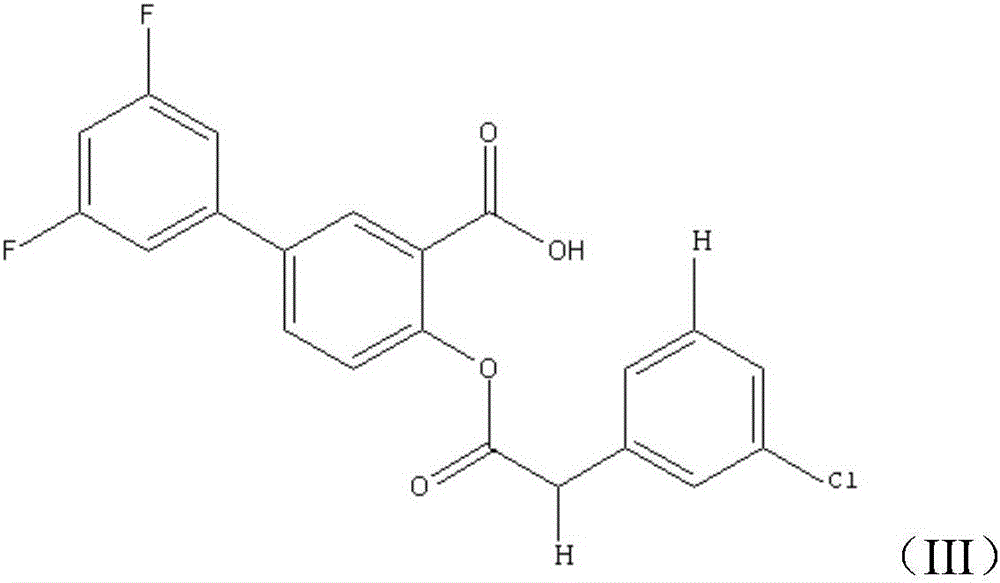
[0030] Step 2. Weigh 8g of diflunisalate and dissolve it in 40mL of solvent, add 4g of N,N-dimethylformamide as a catalyst, then add substituted phenylacetyl chloride, react at 65°C for 8 hours, remove After solvent, obtain phenylacetyl fluorophenyl salicylic acid;
[0031] Step 3. Dissolve 1 g of dichloro-di-methylcymene diruthenium dichloride and 3 g of phenylacetylfluorophenylsalicylic acid in 80 mL of tetrahydrofuran, react at 65° C. for 6 hours, and purify. Instantly.
[0032] Wherein, the ruthenium trichloride hydra...
Embodiment 2
[0037] A preparation method of fluorophenyl ruthenium compound, comprising the following steps:
[0038] Step 1. Dissolve 6g of ruthenium trichloride hydrate and 80mL of γ-terpinene in 200mL of absolute ethanol, heat and reflux and stir for 6 hours, and leave to precipitate to obtain dichloro-dichloro-di-methylisopropyl Benzodiruthenium.
[0039] Step 2: Weigh 12g of diflunisalate and dissolve it in 80mL of solvent, add 8g of N,N-dimethylformamide as a catalyst, then add substituted phenylacetyl chloride, react at 75°C for 12 hours, remove After solvent, phenylacetylfluorophenylsalicylic acid is obtained.
[0040] Step 3. Dissolve 3 g of dichloro-di-methylcymene diruthenium dichloride and 9 g of phenylacetylfluorophenylsalicylic acid in 100 mL of tetrahydrofuran, react at 75° C. for 10 hours, and purify. Instantly.
[0041] Wherein, the ruthenium trichloride hydrate in the step 1 is ruthenium trichloride hydrate with a ruthenium weight content of 45%; the purity of the γ-te...
Embodiment 3
[0046] A preparation method of fluorophenyl ruthenium compound, comprising the following steps:
[0047] Step 1. Dissolve 3g of ruthenium trichloride hydrate and 60mL of γ-terpinene in 120mL of absolute ethanol, heat and reflux and stir for 6 hours, and leave to precipitate to obtain dichloro-dichloro-di-methylisopropyl Benzodiruthenium;
[0048] Step 2. Weigh 10 g of diflunisalate and dissolve it in 60 mL of solvent, add 6 g of N,N-dimethylformamide as a catalyst, then add substituted phenylacetyl chloride, react at 70 ° C for 10 hours, remove After solvent, obtain phenylacetyl fluorophenyl salicylic acid;
[0049] Step 3. Dissolve 2 g of dichloro-di-methylcymene diruthenium dichloride and 6 g of phenylacetylfluorophenylsalicylic acid in 90 mL of tetrahydrofuran, react at 70° C. for 8 hours, and purify. Instantly.
[0050] Wherein, the ruthenium trichloride hydrate in the step 1 is ruthenium trichloride hydrate with a ruthenium weight content of 45%; the purity of the γ-terp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com