Dynamic bond-containing polyurethane material for 3D printing and its preparation method and use
A polyurethane material, 3D printing technology, applied in the direction of additive processing, organic chemistry, etc., can solve problems such as the application of polymers with functional groups that have not been seen, achieve good flexibility and improve mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
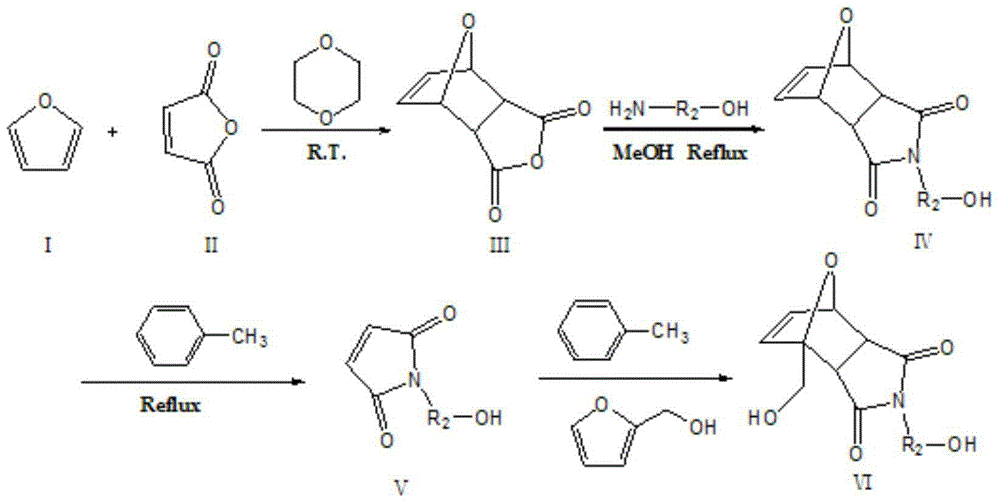
[0040] Dissolve equimolar amounts of furan and maleic anhydride in excess 1,4-dioxane, react at room temperature for 12 hours, smash the precipitate, filter with suction, wash with ether, and dry to obtain product III; product III React with methanol amine in an equimolar amount at a temperature of 65°C in excess methanol for 12 hours, put the reaction mixture into the refrigerator to cool and crystallize, then filter with suction, wash with ether, and dry to obtain the product IV; add the product IV to the excess In toluene, reflux reaction at a temperature of 100°C for 10h, then filter the solution and put it into a refrigerator to cool and crystallize, filter with suction, wash with ether, and dry to obtain the product Ⅴ; equimolar amounts of product Ⅴ and furfuryl alcohol at a temperature of 70°C, in React in excess toluene for 12 hours, mash the precipitate, filter with suction, wash with ether, and dry to obtain product VI, which is a diol containing a Diels-Alder bond; ...
Embodiment 2
[0043] Dissolve equimolar amounts of furan and maleic anhydride in excess 1,4-dioxane, react at room temperature for 18 hours, smash the precipitate, filter with suction, wash with ether, and dry to obtain product III; product III React with ethanolamine in an equimolar amount at a temperature of 68°C in excess methanol for 18 hours, put the reaction mixture in the refrigerator to cool and crystallize, then filter with suction, wash with ether, and dry to obtain product IV; add product IV to excess toluene reflux reaction at a temperature of 105°C for 12h, then filter the solution and put it into a refrigerator for cooling and crystallization, and then obtain the product Ⅴ through suction filtration, washing with ether, and drying; React in toluene for 18 hours, crush the precipitate, filter with suction, wash with ether, and dry to obtain product VI, which is a diol containing Diels-Alder bond;
[0044] Melt 100 parts of toluene diisocyanate and 55 parts of polyethylene adipa...
Embodiment 3
[0046] Dissolve equimolar amounts of furan and maleic anhydride in excess 1,4-dioxane, react at room temperature for 24 hours, smash the precipitate, filter with suction, wash with ether, and dry to obtain product III; product III React with propanolamine in an equimolar amount at a temperature of 71°C in excess methanol for 24 hours, put the reaction mixture in the refrigerator to cool and crystallize, then filter with suction, wash with ether, and dry to obtain product IV; add product IV to excess in toluene at a temperature of 110°C for 14 hours, then filtered the solution and placed it in a refrigerator to cool and crystallize, and then filtered, washed with ether, and dried to obtain the product V; equimolar amounts of the product V and furfuryl alcohol at a temperature of 76°C, React in excess toluene for 24 hours, crush the precipitate, filter with suction, wash with ether, and dry to obtain product VI, which is a diol containing Diels-Alder bond;
[0047] 100 parts of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com