Compositions and methods for suppressing endometrial proliferation
A technology of composition and medicine, applied in the field of compositions for inhibiting endometrial hyperplasia, capable of solving problems such as endometrial hyperplasia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
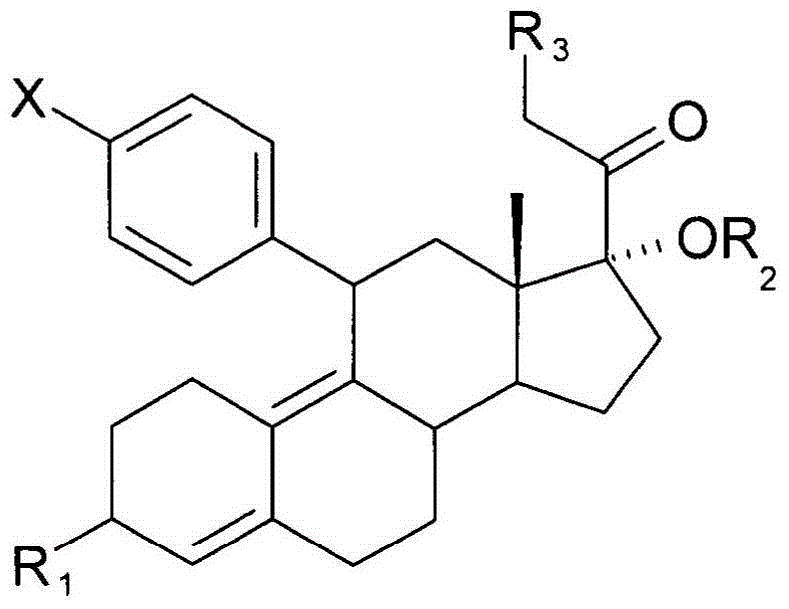
[0120] CLAIMS 1. A method of inhibiting endometrial hyperplasia, said method comprising administering to a woman in need thereof an effective amount of a composition comprising a progesterone antagonist.
[0121] 2. The method of embodiment 1, wherein the woman in need thereof is a woman with endometriosis.
[0122] 3. The method of embodiment 1, wherein the composition is administered simultaneously, separately or sequentially with the estrogen or the selective estrogen receptor modulator as part of hormone replacement therapy.
[0123] 4. The method of embodiment 1, wherein the binding affinity of the progesterone antagonist for the progesterone receptor is at least 1.5 times the binding affinity of the progesterone antagonist for the glucocorticoid receptor.
[0124] 5. The method of embodiment 1, wherein progesterone levels in the female do not increase significantly following administration of the composition.
[0125] 6. The method of embodiment 1, wherein estrogen leve...
Embodiment 1
[0180] Example 1. The preparation of the present invention can be made into tablets.
[0181] In order to obtain tablets for use in the practice of the present invention, the following components can be compressed together in a tablet press:
[0182]
[0183] In order to obtain bilayer tablets for use in the practice of the present invention, the following components can be compressed together in a tablet press:
[0184]
[0185] In order to obtain tablets comprising an antiestrogenic substance for use in the practice of the invention, for example, the following components can be compressed together in a tablet press:
[0186]
[0187] To obtain an oily preparation for the practice of the invention, for example, the following ingredients may be mixed together and added to ampoules:
[0188] 100.0mg CDB-4124
[0189] 343.4mg castor oil
[0190] 608.6mg Benzyl Benzoate
Embodiment 2
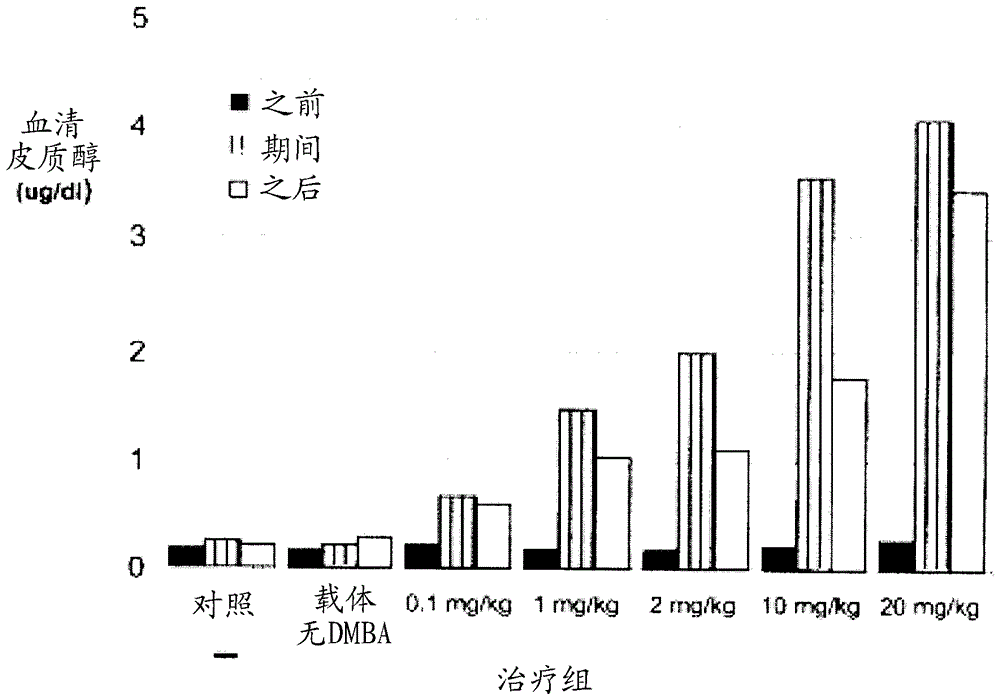
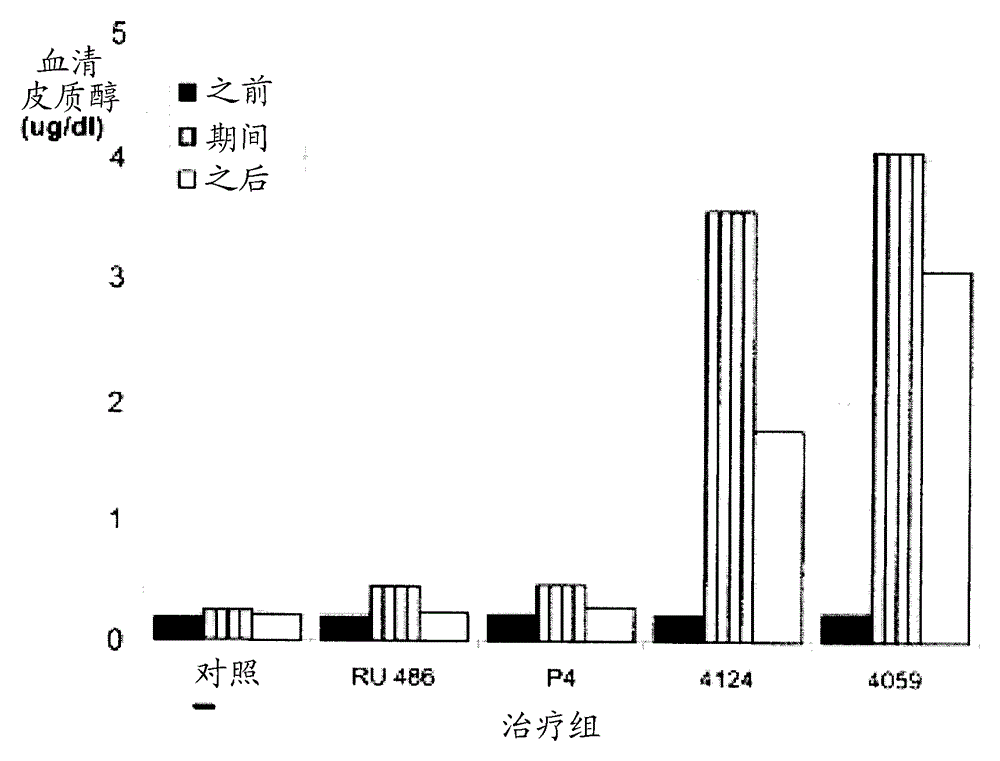
[0191] Example 2. Compounds of the invention may have only weak anti-glucocorticoid receptor binding activity.
[0192] The ability of some antiprogestogens to bind the rabbit progesterone receptor (rbPR) and glucocorticoid receptor (rbGR) was tested in a receptor binding assay. Briefly, uterus or thymus containing Cytoplasm of PR or GR. For PR binding, mix cytoplasm with 6 nM 1,2-[ 3 H] progesterone (50.0Ci / mmol) was incubated together, and a competitor was added at a concentration of 2-100 nM. For GR binding, mix cytoplasm with 6 nM 6,7-[ 3 H]-dexamethasone (40Ci / mmol) was incubated together, and the competitor was added at a concentration of 2-100 nM. After overnight incubation at 4°C, bound and unbound [ 3 H] Steroids. will contain [ 3 The supernatant of the H]-steroid receptor complex was decanted into vials containing 4 ml of Optifluor (Packard Instrument Co.), vortexed, equilibrated in a liquid scintillation counter for 30 minutes, and then counted for 2 minutes....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com