Cd20-binding immunotoxins for inducing cellular internalization and methods using same
A cell and toxin technology, applied in the field of CD20 binding protein, can solve the problem of insufficient CD20 internalization efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0214] The following examples illustrate certain embodiments of the invention. However, it should be understood that these examples are for illustrative purposes only, and are not intended and should not be construed as fully limiting the state and scope of the present invention. The described embodiments are performed using standard techniques, which are well known and conventional to those skilled in the art, except where otherwise described in detail.
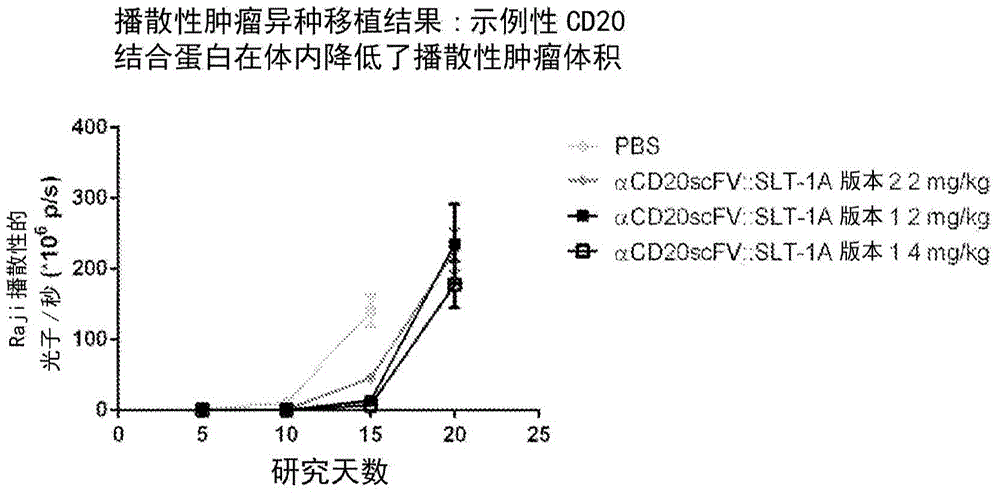
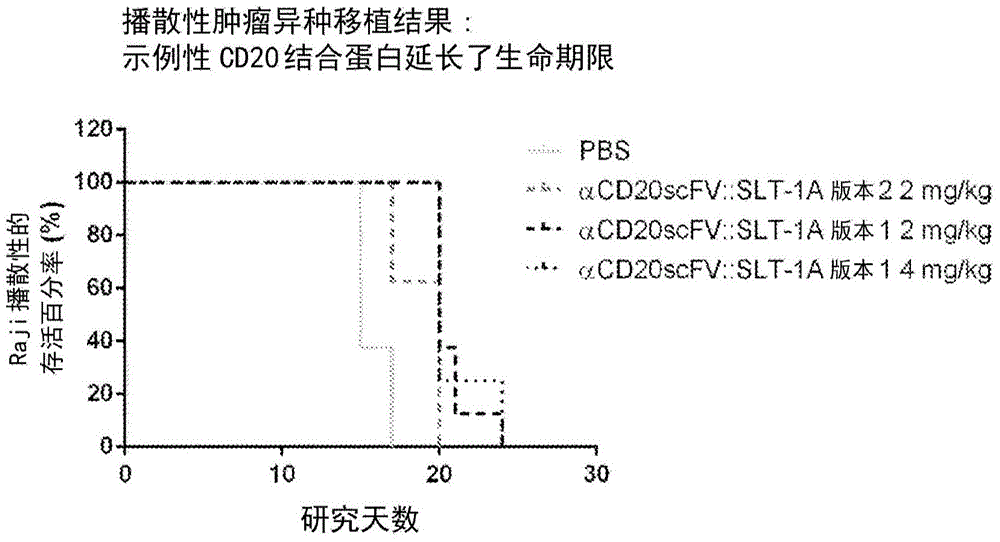
[0215] The following examples show the ability of exemplary CD20 binding proteins to selectively kill cells expressing CD20 on their cell surface. An exemplary CD20 binding protein binds to the extracellular antigen on CD20 expressed by the targeted cell type and enters the targeted cell. The internalized CD20 binding protein provides its Shigella toxin effector region with a route to the cytosol to inactivate ribosomes and subsequently cause apoptotic death of targeted cells. Therefore, after the CD20 binding protein forms a...
Embodiment 2
[0223] Example 2-Determination of the dissociation constant of an exemplary CD20 binding protein (K D )
[0224] The cell binding characteristics of both αCD20scFv::SLT-1ACD20 binding protein version 1 and 2 were determined by fluorescence-based flow cytometry. Each sample contains 0.5x10 6 CD20-expressing cells (Raji(CD20+)) or non-CD20-expressing cells (BC1(CD20-)) and 100 μL of CD20 binding protein in various dilutions in Hyclone 1XPBS (Fisher Scientific, Waltham, MA) + 1% BSA ( Incubate in Calbiochem, SanDiego, CA, USA) (hereinafter referred to as "1XPBS+1%BSA") at 4°C for 1 hr. The highest concentration of CD20 binding protein is selected to cause saturation of the reaction. The cells were washed twice with 1XPBS + 1% BSA. Combine cells with 100μl containing 0.3ug anti-Strep mAb-FITC (#A01736-100, Genscript, Piscataway, NJ, USA) in 1XPBS+1%BSA was incubated at 4°C for 1hr. The cells were washed twice with 1XPBS + 1% BSA, suspended in 200 μl of 1XPBS, and subjected to flo...
Embodiment 3-
[0228] Example 3-Determining the half-maximal inhibitory concentration (IC 50 )
[0229] Use The cell-free in vitro protein translation assay of the QuickCoupled Transcription / Translation kit (L1170 Promega Madison, WI, USA) was used to determine the ribosome inactivation capacity of both αCD20scFv::SLT-1ACD20 binding protein versions 1 and 2. The kit includes Luciferase T7 Control DNA (LuciferaseT7ControlDNA) (L4821PromegaMadison, WI, USA) and QuickMasterMix. Prepare the ribosome activity reaction according to the manufacturer's instructions.
[0230] Prepare a series of 10-fold dilutions of the αCD20scFv::SLT-1A version to be tested in a suitable buffer and generate a series of identical TNT reaction mixture components for each dilution. Combine each sample in the dilution series of αCD20scFv::SLT-1A protein with each of the TNT reaction mixture and the luciferase T7 control DNA. The test sample was incubated at 30°C for 1.5 hours. After incubation, luciferase assay reagent ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com