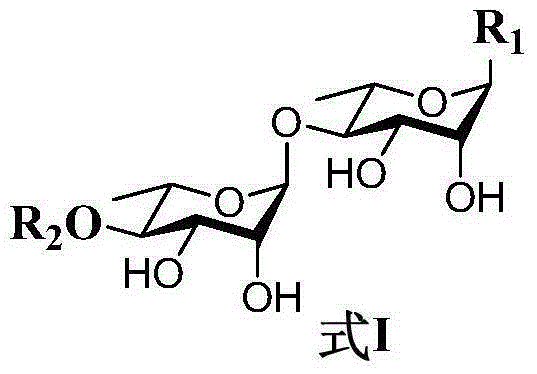
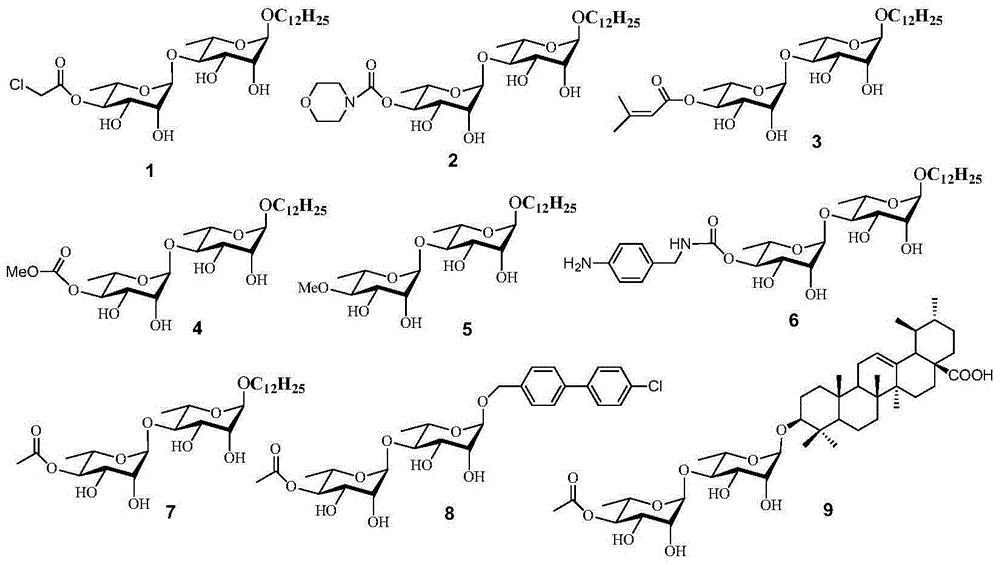
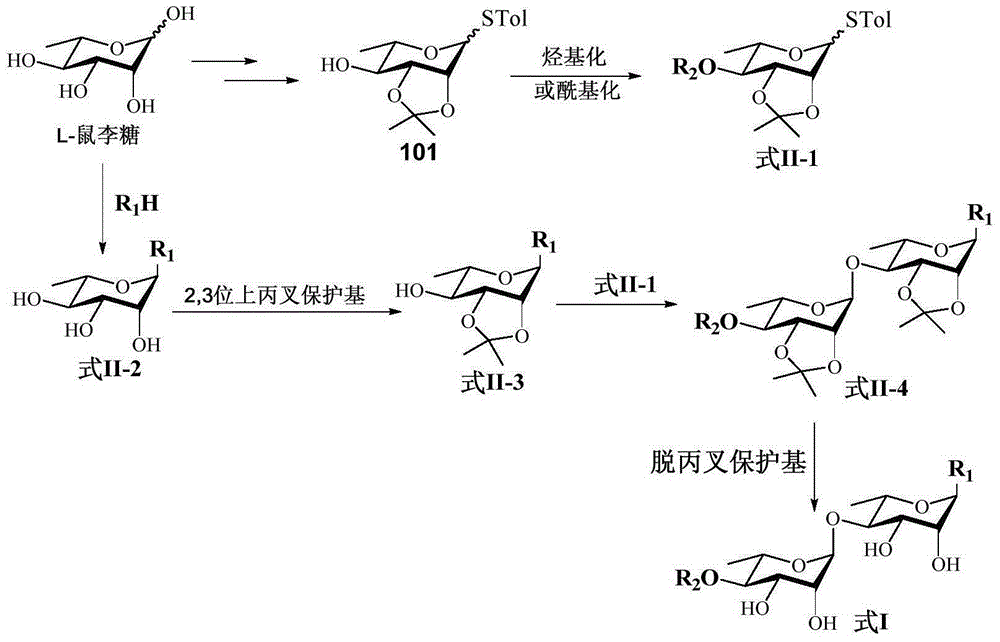
Rhamnoside compound and application thereof as medicament for anti-multidrug resistant tumor
An anti-tumor drug, rhamnoside technology, applied in the direction of anti-tumor drugs, steroids, sugar derivatives, etc., can solve the problems of limited drug delivery and low water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Preparation of monosaccharide glucosinolate donors 71-78
[0059] (1):
[0060] Weigh 3-methyl-2-butenoic acid (645mg, 6.44mmol) dissolved in dry CH 2 Cl 2 (30mL), add DCC (499mg, 2.42mmol), DMAP (39mg, 0.322mmol), stir at room temperature for 5 minutes, add compound 101 (500mg, 1.61mmol), stir the reaction at room temperature until TLC detects that compound 101 disappears, filter , After concentration under reduced pressure, through silica gel column chromatography (petroleum ether / EtOAc=10 / 1), compound 71 (531 mg; 84%) and its β-isomer were obtained;
[0061] Compound 71: 1 HNMR (400MHz, CDCl 3 ):δ7.38-7.34(m,2H),7.13(d,J=7.9Hz,2H,),5.71(s,1H),5.69(s,1H),4.96(dd,J=9.9,7.8Hz ,1H),4.35(dd,J=5.3,0.5Hz,1H),4.23(dd,J=8.0,5.5Hz,1H),4.21–4.17(m,1H),2.33(s,3H),1.93( s,3H),1.91(d,J=1.0Hz,3H),1.58(s,3H),1.35(s,3H),1.13(d,J=6.3Hz,3H).
[0062] Monosaccharide glucosinolate donors 72 and 73 can be prepared according to the above-mentioned similar method or the m...
Embodiment 2
[0078] Example 2 Preparation of Monosaccharide Acceptors 81-90
[0079]
[0080] Weigh L-rhamnose (2.0g, 10.98mmol) and n-hexadecanol (13.31g, 54.89mmol) with 1 crystal water and heat to 80°C, add TfOH–SiO 2 (274mg, 2mmol / g), after stirring and reacting for 5-8h, pour the reaction mixture into a (8cm×2cm) silica gel column while hot, and first use CH 2 Cl 2 Methane elution reclaims unreacted n-hexadecanol, and then elutes with EtOAc to obtain hexadecyl rhamnoside, which is dissolved in 50 mL of acetone, and 2,2-dimethoxypropane (2.1 mL, 16.47 mmol ), a catalytic amount of p-toluenesulfonic acid, stirred and reacted at room temperature for 0.5 h, concentrated, and subjected to silica gel column chromatography (petroleum ether / EtOAc=5 / 1) to obtain compound 89 (3.76 g, 80%) and its β-isomer;
[0081] Compound 89: 1 HNMR (400MHz, CDCl 3 ):δ4.94(s,1H),4.14–4.08(m,2H),3.69(dt,J=9.2,6.7Hz,2H),3.45–3.38(m,2H),2.47(broads,1H), 1.68–1.52(m,4H),1.53(s,3H),1.36(s,3H),1.31–1.24(m,2...
Embodiment 3
[0093] The preparation of embodiment 3 intermediate compound 111-134
[0094] (1): Weigh compound 85 (100mg, 0.268mmol) and compound 73 (138mg, 0.349mmol) and dissolve in 5mL dry dichloromethane, add appropriate amount MS, under the protection of argon, under ice bath, add NIS (90mg, 0.402mmol), TfOH (5μL), stir the reaction for 0.5 ~ 1h, filter, wash with 20mLCH 2 Cl 2 Diluted sequentially with 5% Na 2 S 2 o 3 , saturated NaHCO 3 , washed with saturated NaCl, dried over anhydrous sodium sulfate, concentrated, and subjected to silica gel column chromatography (petroleum ether / EtOAc=8 / 1) to obtain compound 111 (155 mg, 90%);
[0095] Compound 111: 1 HNMR (400MHz, CDCl 3 ):δ5.64(s,1H),4.98–4.93(m,2H),4.21(dd,J=7.1,5.7Hz,1H),4.18–4.13(m,2H),4.10(d,J=5.6 Hz,1H),3.77(dq,J=12.7,6.3Hz,1H),3.70–3.62(m,2H),3.57(dd,J=9.9,7.3Hz,1H),3.42(dt,J=9.6, 6.5Hz, 1H), 3.27(d, J=16.4Hz, 1H), 3.17(d, J=16.4Hz, 1H), 2.36(s, 6H), 1.61–1.56(m, 5H), 1.54(s, 3H), 1.34(s, 3H), 1.33(s, 3H), 1....
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap