Cartilage-binding fusion proteins
A technology of fusion protein and cartilage tissue, applied in the direction of fusion polypeptide, peptide/protein component, animal/human protein, etc., can solve the problem of unmet need for effective treatment of joint degenerative diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0164] Example 1: Methods for protein expression and purification
[0165] Method for transient production of protein in 293F cells
[0166] The nucleic acid encoding the desired protein sequence was cloned into the pCep4 vector (Invitrogen) using standard recombinant DNA technology. The transformed NEB5-α competent Escherichia coli (New England Biolabs (New England Biolabs) was selected in 1 L of Luria Broth in the presence of ampicillin (ampicillin) selection at 37°C under shaking at 2000 rpm). )) Cultivate overnight to expand the cloned vector. The cells are harvested and used by spinning at 5000g for 20 minutes Plasmid MegaKit (Qiagen) to extract vector DNA from bacterial pellets. By adding 20 mL of 200 mM L-glutamine (source?) and 10 mL of 10% Pluronic F-68 to 1 L of F17 medium ( ) To prepare 293F cell culture medium. For transient transfection, at 37°C and 5% CO 2 Let 1L of 293F cells grow to a density of 1.5-2 million cells / ml. 1 mg of total protein and 2.5 mL of pol...
Example Embodiment
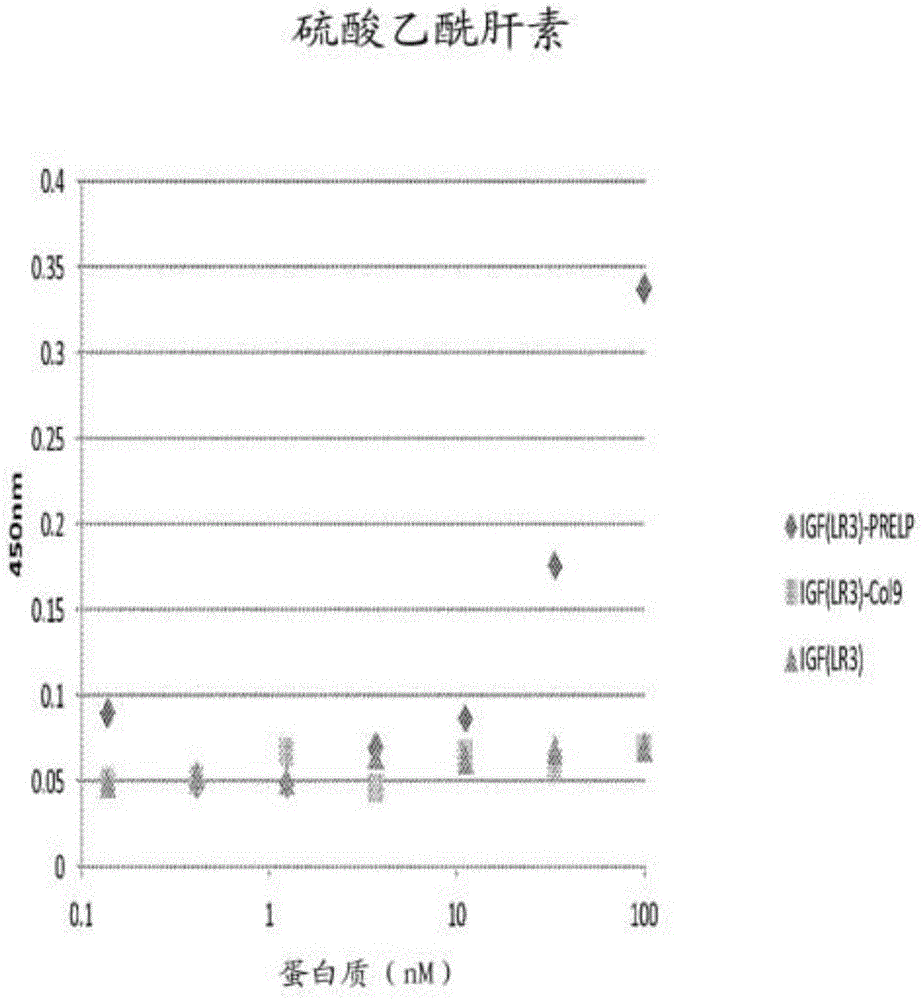
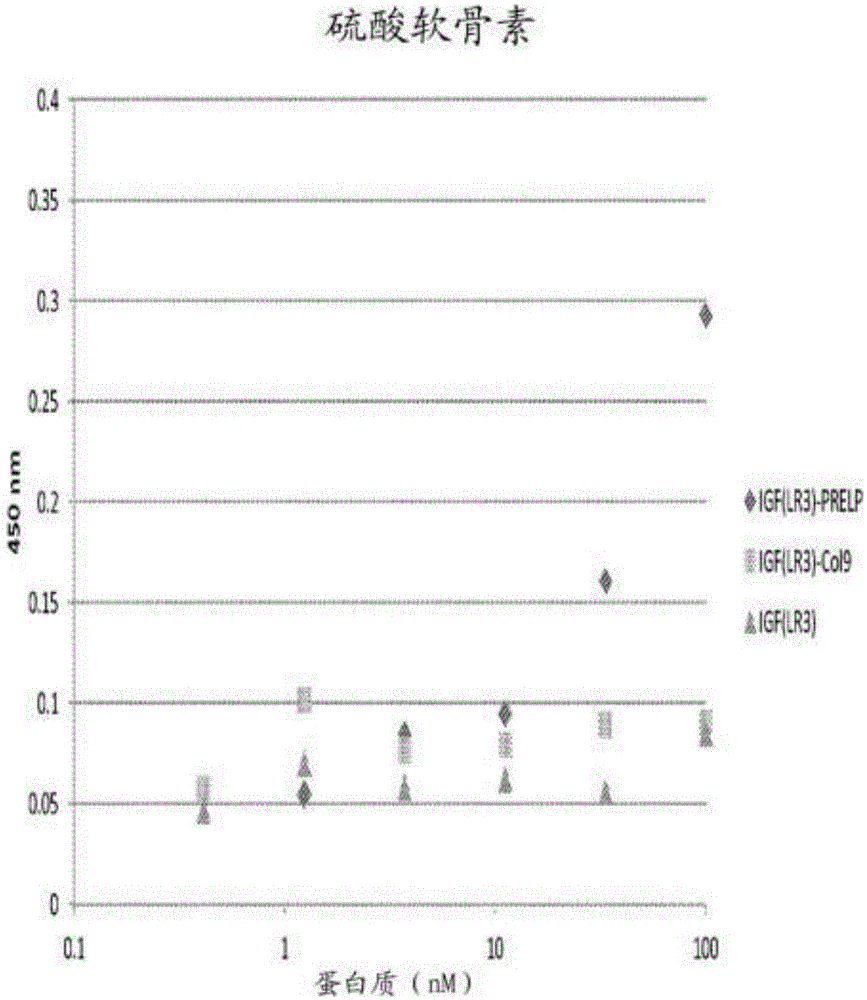
[0178] Example 2: Combination of fusion protein with heparan sulfate and chondroitin sulfate
[0179] The specificity of the binding of the fusion protein to the polysaccharides heparan sulfate and chondroitin sulfate can be determined by measuring the ability of the protein to bind to the polysaccharide coated on the ELISA plate. Coat the heparin binding plate (BDBiosciences) with 50μl of heparan sulfate or chondroitin sulfate (2-10μg / mL). ) And incubate overnight at room temperature. The plate was washed with PBS and blocked with 250 μl of PBS containing 0.2% gelatin at 37°C for 1 hour. The plate was then washed with PBS and patted dry. A dilution series of 50 μl protein was added to the wells and incubated at 37°C for 2 hours. The protein dilution series started at 100 nM and included 10 additional three-fold dilutions in PBS, 0.2% gelatin, and a blank control (only PBS, 0.2% gelatin). After washing the plate in PBS, 50 μl of anti-human IGF-1 (Abcam) (in PBST, 1:250) was ...
Example Embodiment
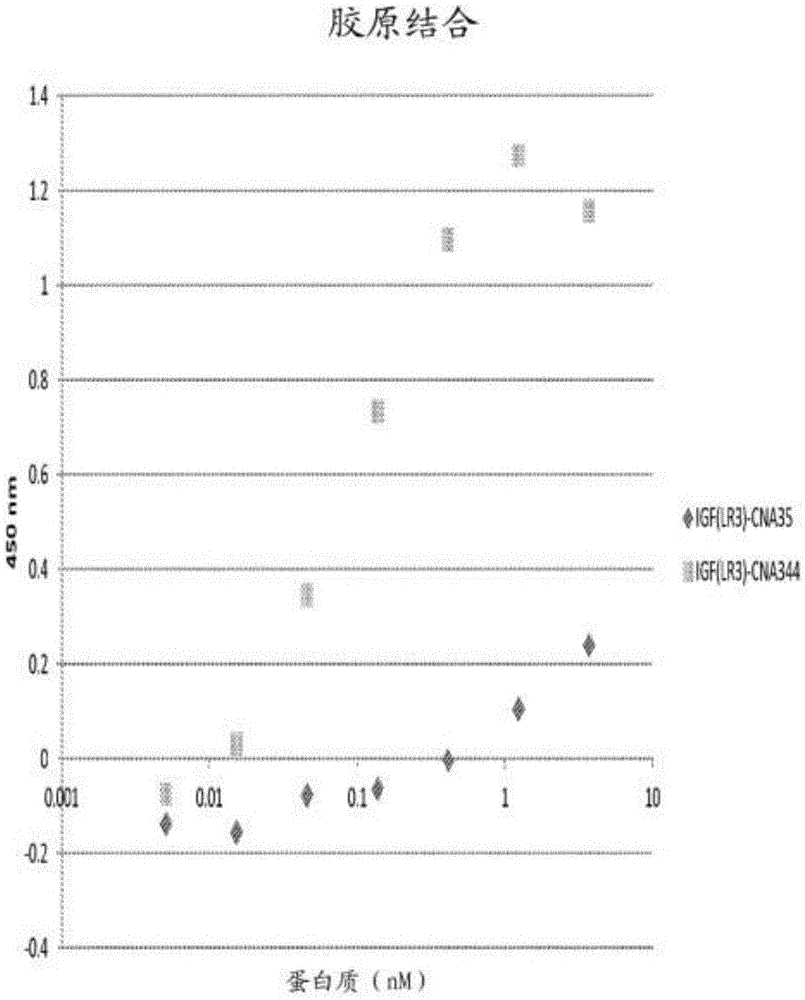
[0181] Example 3: Binding of fusion protein to collagen II
[0182] The specificity of the protein to type II collagen can be determined by measuring the ability of the protein to bind to the collagen coated on the ELISA plate. will The 96-well plate was coated with 100 μl of type II collagen (Chondrex Products, Redmond, WA) at 4° C. with 1× the provided buffer overnight. The plate was washed with PBS, 0.05% Tween-20 (PBST) and blocked with 100 μl of protein-free blocking buffer (Pierce, Thermo Scientific) for 1 hour at room temperature. The plate was then washed with PBST and patted dry. A dilution series of 50 μl protein was added to the well and incubated for 1 hour at room temperature. The protein dilution series started at 100 μM and included 10 additional three-fold dilutions in PBS and a blank control (PBS). After the plate was washed in PBS, 50 μl of anti-human IGF-1 (Abcam) (in PBST, 1:250) was added and the plate was incubated for 1 hour at room temperature with ro...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap