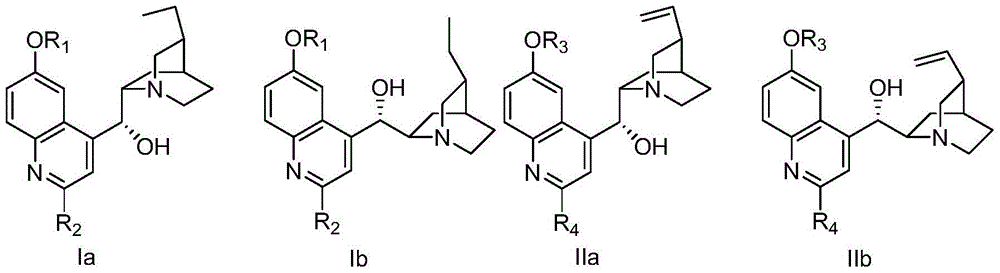
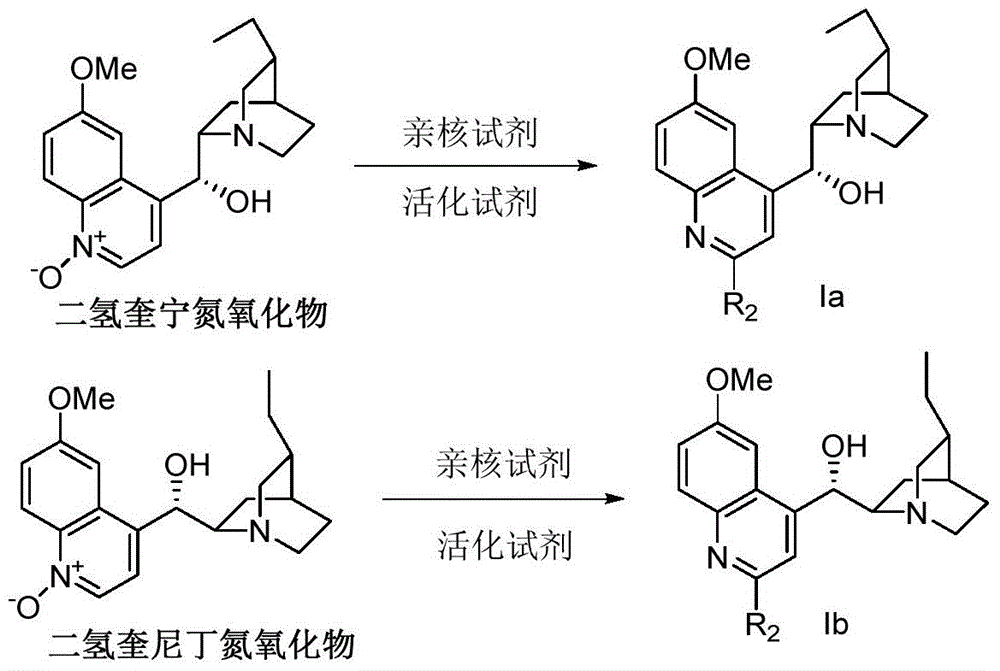
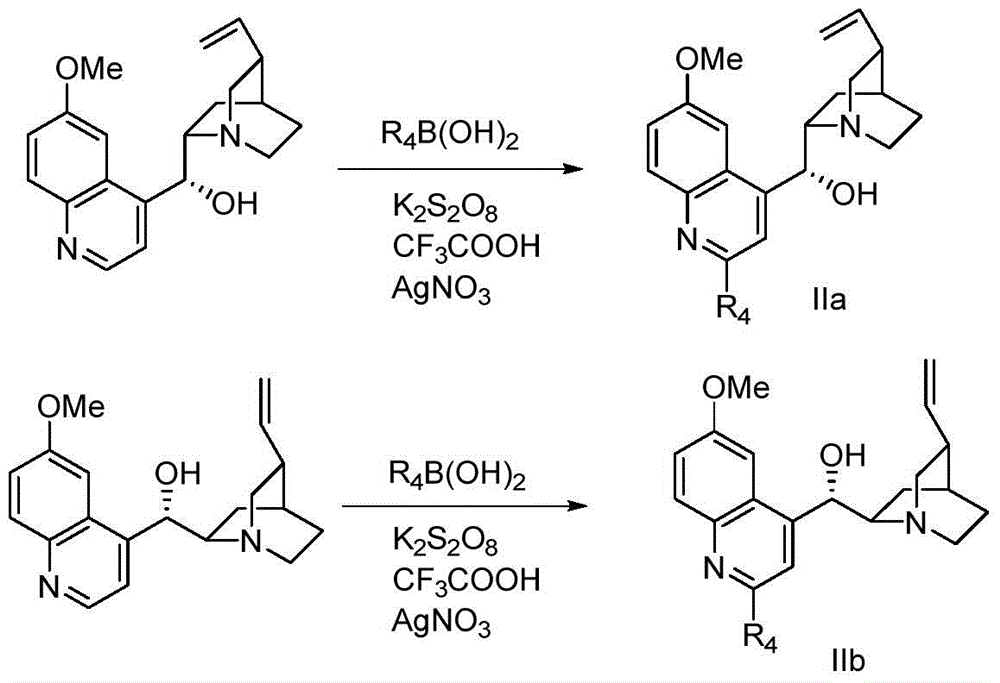
Method for preparing chiral α-hydroxy-β-dicarbonyl compound with novel cinchona base c-2` derivative as catalyst
A technology of dicarbonyl compounds and cinchona base, which is applied in the field of efficient preparation of chiral α-hydroxy-β-dicarbonyl compounds to achieve high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: 2 '-bromo-dihydroquinine preparation (formula Ia, R 2 for Br, R 1 For Me): Weigh 3.26 g of dihydroquinine, dissolve in 45 mL of chloroform, and cool to 0°C. Subsequently, 5.08 g of m-chloroperoxybenzoic acid with a mass fraction of 85% was added and stirred for half an hour. Subsequently, it was raised to room temperature, and stirring was continued for 3 hours until the reaction was complete. A 10% aqueous NaOH solution was added to the reaction solution until the pH of the aqueous layer=10. The aqueous phase was extracted 5 times with chloroform / methanol=10:1 mixed solution. The organic phases were combined and dried over anhydrous sodium sulfate. The solvent was suspended to dry to obtain dihydroquinine bis-N-O compound (3.55 g, 99% yield) as light yellow solid. The crude product was directly used in the next reaction. Weigh 2.60gNaHSO 3, add 20 mL of 1mol / L HCl, and stir for one hour. Subsequently, the temperature was lowered to 0°C. The dihyd...
Embodiment 2
[0044] Embodiment 2: 2'-bromo-6'-hydroxyl-dihydro cinchonidine (formula Ia, R 2 for Br, R 1 For the preparation of H):
[0045] Weigh 2'-bromo-dihydroquinine (0.48g) and place it in a 25mL three-necked flask, and add 5mL of 40% mass fraction of hydrobromic acid. The mixture was reacted at 110°C for 16 hours. Cool down and adjust pH=9 using solid NaOH. The mixture was extracted 4 times with dichloromethane / methanol=15:1 mixture, the organic phases were combined, dried over anhydrous sodium sulfate, concentrated, and separated by column chromatography (40% ethyl acetate, 15% methanol, 2% triethylamine , 43% petroleum ether) to obtain 0.39 g of 2`-bromo-6`-hydroxyl-dihydrocinchonidine, with a yield of 85%. 1H NMR (500MHz, Methanol-d4) δ7.83 (dd, J = 9.0, 1.5Hz, 1H), 7.68 (s, 1H), 7.33 (ddd, J = 9.2, 2.6, 1.2Hz, 1H), 7.24 ( d,J=2.6Hz,1H),5.47(d,J=3.3Hz,1H),3.70(dtd,J=8.1,5.3,2.5Hz,1H),3.21–3.03(m,2H),2.74(qd ,J=7.4,3.4Hz,3H),2.49–2.36(m,1H),1.97–1.79(m,3H),1.62–1.49(m,2H),1....
Embodiment 3
[0046] Embodiment 3: 2 '-chloro-dihydroquinine (formula Ia, R 2 for Cl, R 1 Preparation of Me): at 0°C, weigh 0.34 g of dihydroquinine mono-N-O compound, add 5 mL of chloroform, and add 0.61 g of phosphorus oxychloride under nitrogen protection. After stirring for 30 minutes, the temperature was raised to 70° C., and the reaction was refluxed for 2.5 hours. After the reaction, 15 mL of ice water was added, and the pH was adjusted to 10 with ammonia water. The mixture was extracted four times with dichloromethane, and the combined organic phases were dried over anhydrous sodium sulfate and concentrated. Column chromatography separation (40% ethyl acetate, 5% methanol, 2% triethylamine, 53% petroleum ether) gave 0.30 g of 2'-chloro-dihydroquinine with a yield of 83%. 1 H NMR (500MHz, Methanol-d 4 )δ8.00–7.82(m,1H),7.64(d,J=2.9Hz,1H),7.54–7.34(m,2H),5.55–5.44(m,1H),4.12–3.88(m,3H) ,3.71(s,1H),3.15(s,2H),2.97(q,J=7.3Hz,1H),2.77(s,1H),2.47(d,J=12.4Hz,1H),1.99–1.76( m,3H), 1.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com