Reproducible RNA vaccine for control of toxoplasmosis and construction method and application thereof
A technology for toxoplasmosis and a construction method, applied in the fields of immunology and molecular biology, can solve the problems of difficult population prevention, reduced immune effect, inability to apply large-scale vaccination and prevention, etc., to enhance humoral immunity and cellular immune response. , prolong the survival time, reduce the effect of the number of cysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0055] Below in conjunction with embodiment, the present invention is further described; Following embodiment is illustrative, not limiting, can not limit protection scope of the present invention with following embodiment.
[0056] The raw materials used in the present invention, unless otherwise specified, are conventional commercially available products; the methods used in the present invention, unless otherwise specified, are conventional methods in the art.
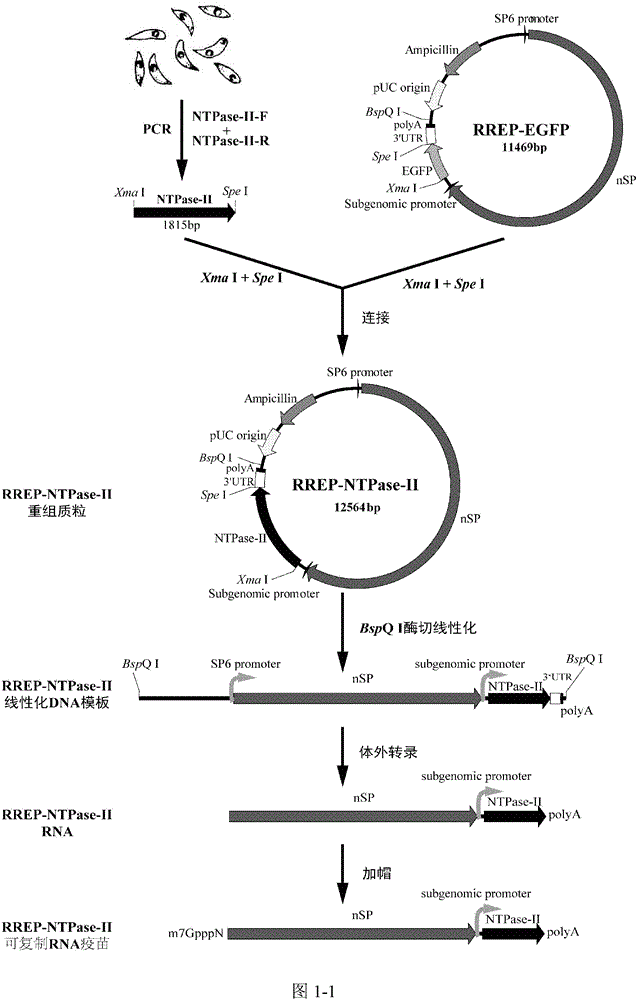
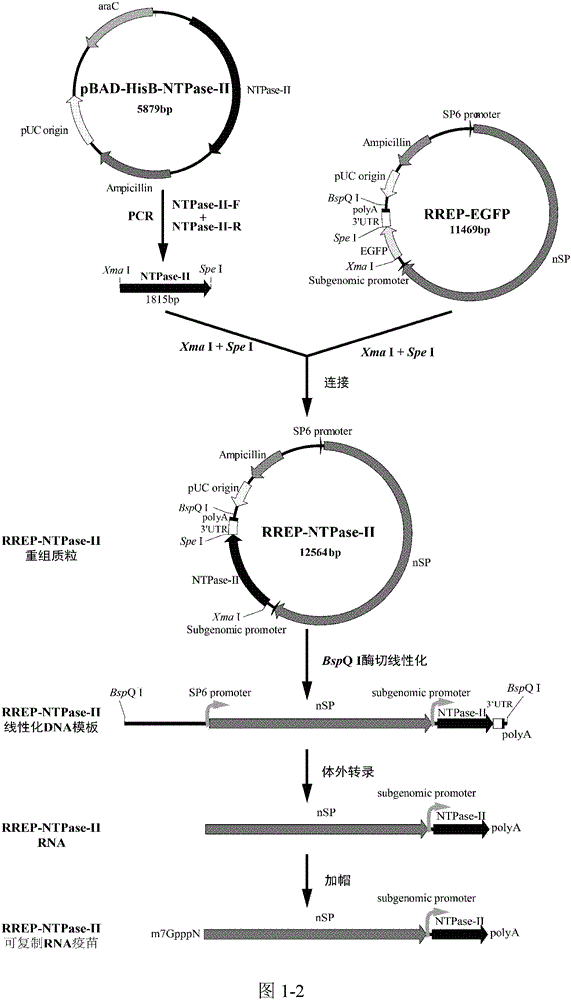
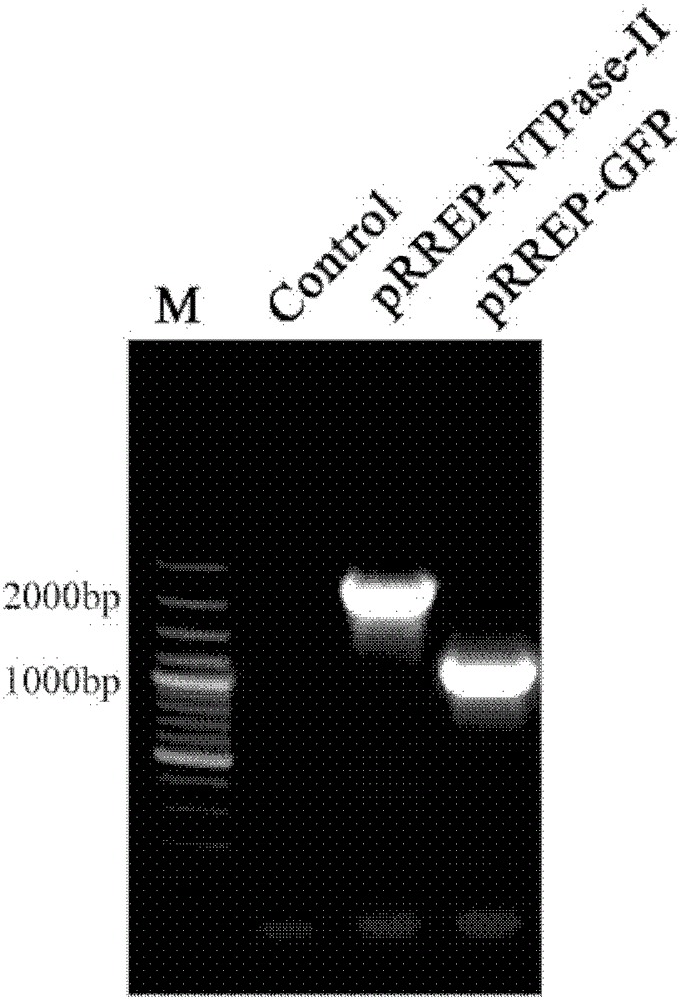
[0057] A replicable RNA vaccine for preventing acute and chronic toxoplasmosis in humans or animals, inserting the toxoplasma antigen gene NTPase-II into the alphavirus vector RREP to obtain the recombinant plasmid pRREP-NTPase-II, before immunization, the After the recombinant plasmid is transcribed, the RNA vaccine is obtained. Since the plasmid contains alphavirus replicase, it can self-replicate in the cytoplasm and become replicative RNA.
[0058] For the vaccine of the present invention, the way to evaluate th...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap