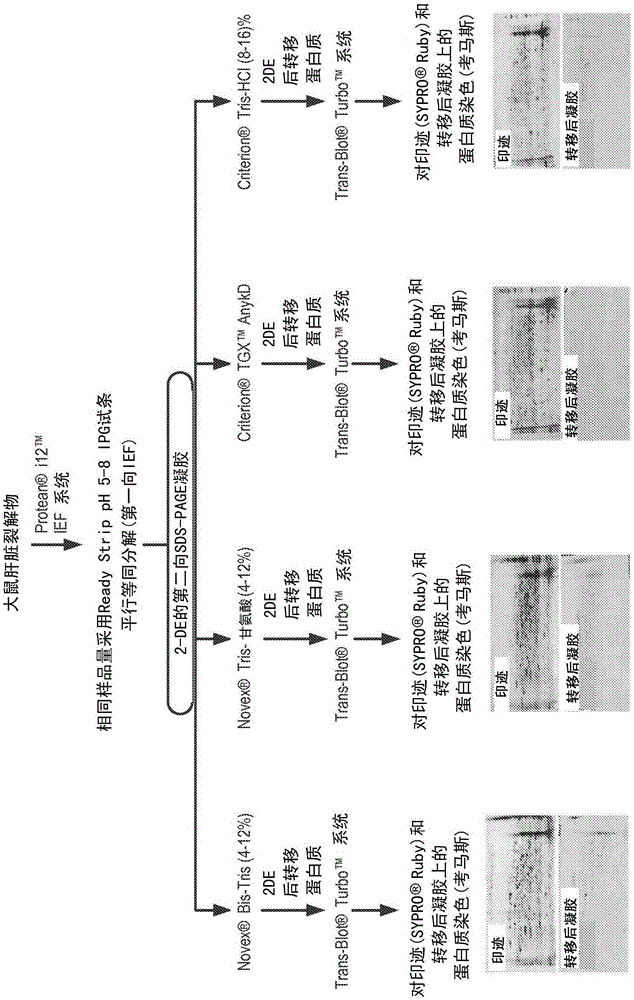
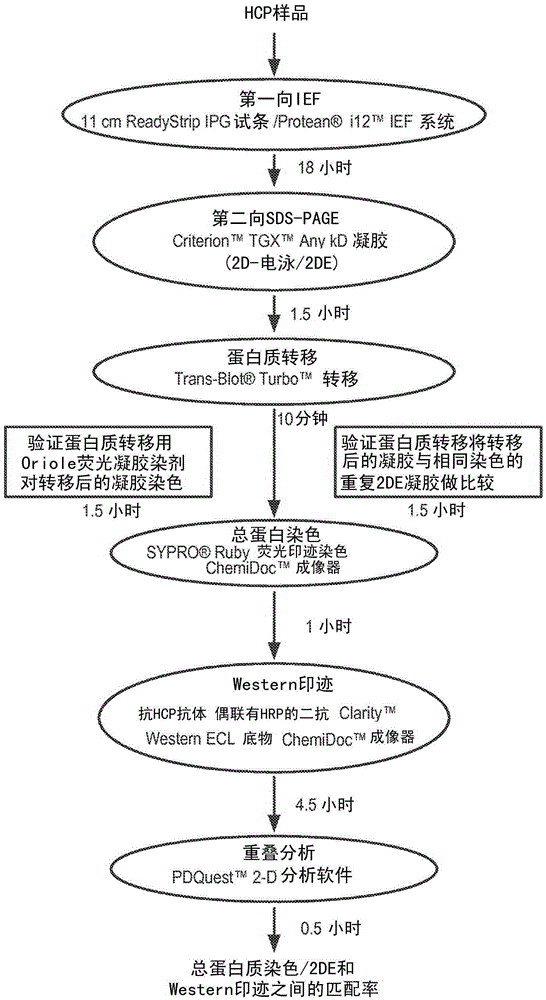
Hcp antiserum validation using a non-interfering protein stain
一种非干扰性、蛋白质的技术,应用在采用非干扰性蛋白质染剂的HCP抗血清验证领域,能够解决匹配的指定复杂、不正确、模糊匹配等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] "Polyclonal antibody" refers to a preparation of antibodies, raised against a single antigen, with varying binding specificities and affinities. "Polyclonal antibody cocktail" refers to a mixture of polyclonal antibodies, each raised against a single antigen. Alternatively, "polyclonal antibody mixture" may refer to a mixture of polyclonal antibodies derived from the serum of an animal vaccinated with the antigen mixture. For example, a "polyclonal antibody mixture" may refer to a mixture of polyclonal antibodies obtained from the serum of an animal that has been inoculated with a preparation comprising host cell proteins.
[0045] A "label" or "detectable moiety" is a composition detectable by spectroscopic, photochemical, biochemical, immunochemical, chemical or other physical means. For example, available markers include 32 P, fluorescent dyes, electron-dense reagents, enzymes (e.g., commonly used in ELISAs), biotin, digoxin, or haptens and proteins or other entiti...
Embodiment 1
[0084] introduction
[0085] Biologic drugs face particular supervisory and technical requirements due to their origin / expression in genetically engineered host cells, their underlying physicochemical properties, and the complex purification processes applied in their production. One of these requirements is the accurate monitoring and efficient removal of process-derived impurities such as host cell proteins (HCP), host cell-derived DNA / RNA, viruses, cell culture media, chromatography leachables, etc. (1). Among the different impurities, accurate monitoring of HCP may be the most difficult, because the proteome of the expression system consists of thousands of different proteins, some of which can copurify with the biological substance drug. Therefore, HCP monitoring methods must be multiple analyte assays with the ability to detect most protein impurities that may be present in any batch of drug substance (2,3).
[0086] Enzyme-linked immunosorbent assay (ELISA) is one of...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap