A kind of α-amylase and its coding gene and application
A technology of amylase and gene, applied in the field of α-amylase and its coding gene and application, can solve the problem that α-amylase does not meet the requirements of industrial application, and achieve good industrial application prospects, high optimum reaction temperature, thermal good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The amplification of embodiment 1α-amylase gene
[0033] 1.1 Strains and their cultivation
[0034] The Antarctic low-temperature bacterium Geomycespannorum (bacteria identification NCBI accession number: JF20026, which has been deposited in CCTCC, number AF2014016) was donated from the cooperation project of the Faculty of Biological Sciences, University of Malaysia.
[0035] Wash G. pannorum spores from the 10-day-cultivated PDA slant with sterile water, inoculate liquid medium, and culture at 20°C for 7 days to collect mycelia for genome extraction: inoculate milk powder solid medium with a toothpick, and culture at 20°C Mycelia were collected after 5 days for total RNA extraction.
[0036] 1.2 Genome Extraction
[0037] Follow the instructions of the Omega Fungal Genome Extraction Kit.
[0038] 1.3 Total RNA extraction
[0039] Follow the instructions of the Takara RNA extraction kit.
[0040] 1.4 cDNA first-strand synthesis
[0041] Using the extracted total ...
Embodiment 2
[0068] Embodiment 2 Contains the construction of the Aspergillus oryzae recombinant expression vector of α-amylase gene and its transformation
[0069] 2.1 Primer design
[0070] SKA1f: 5' CTAGCTAGCTAG ATGTTTTTCAACTGCCCTGC 3'
[0071] SKA1r: 5' TCCCCCGGGGGA TCAAGGGCAATAGCTGCCCT 3'
[0072] 2.2 Construction of recombinant expression vector
[0073] Use the pSKNHG with the open reading frame of the α-amylase gene as a template, and use the primers shown in 2.1 to perform PCR amplification; double-digest the PCR product with NheI and SmaI, and then express it with Aspergillus oryzae that has also been double-digested with NheI and SmaI The vector pSKNHG was ligated and screened to obtain a recombinant plasmid (pSKNHGA1).
[0074] 2.3 Preparation of Aspergillus oryzae Competent Cells
[0075] Aspergillus oryzae slant spores were washed with sterile water, inserted into liquid medium, and cultured overnight.
[0076] When a large number of tiny mycelia appear in the medium, st...
Embodiment 3
[0085] Induced expression and purification of embodiment 3 Aspergillus oryzae recombinant bacteria
[0086] 3.1 Pick a single clone of the recombinant bacteria on the MM plate described in 2.5, inoculate the dextrin-induced liquid medium, and culture at 20°C and 200 rpm for 3 to 5 days.
[0087] 3.2 Collect the supernatant by suction filtration through the membrane, measure the enzyme activity, determine the protein concentration, and conduct protein electrophoresis.
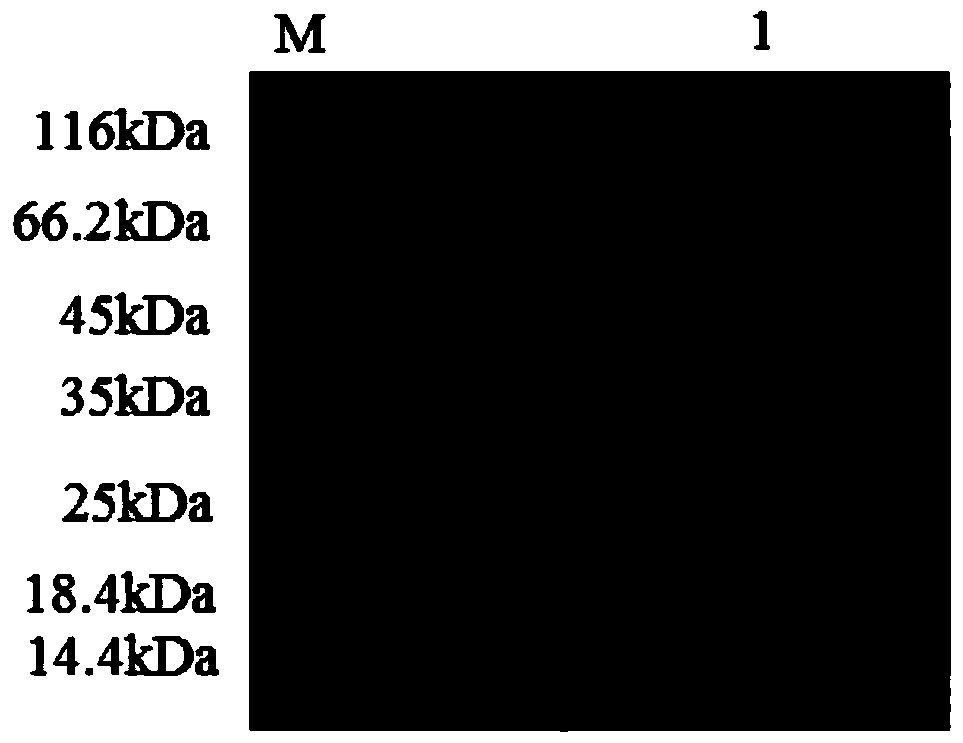
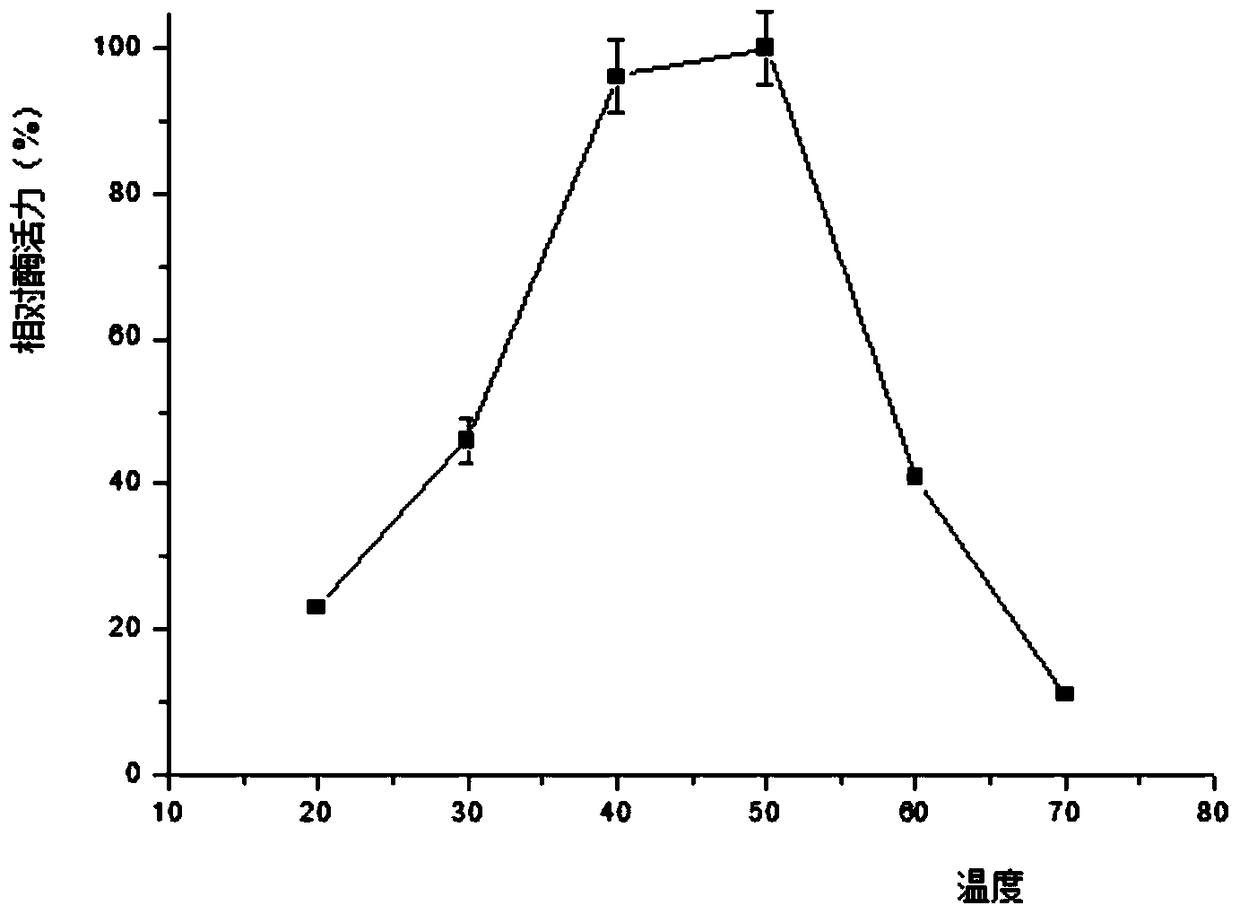
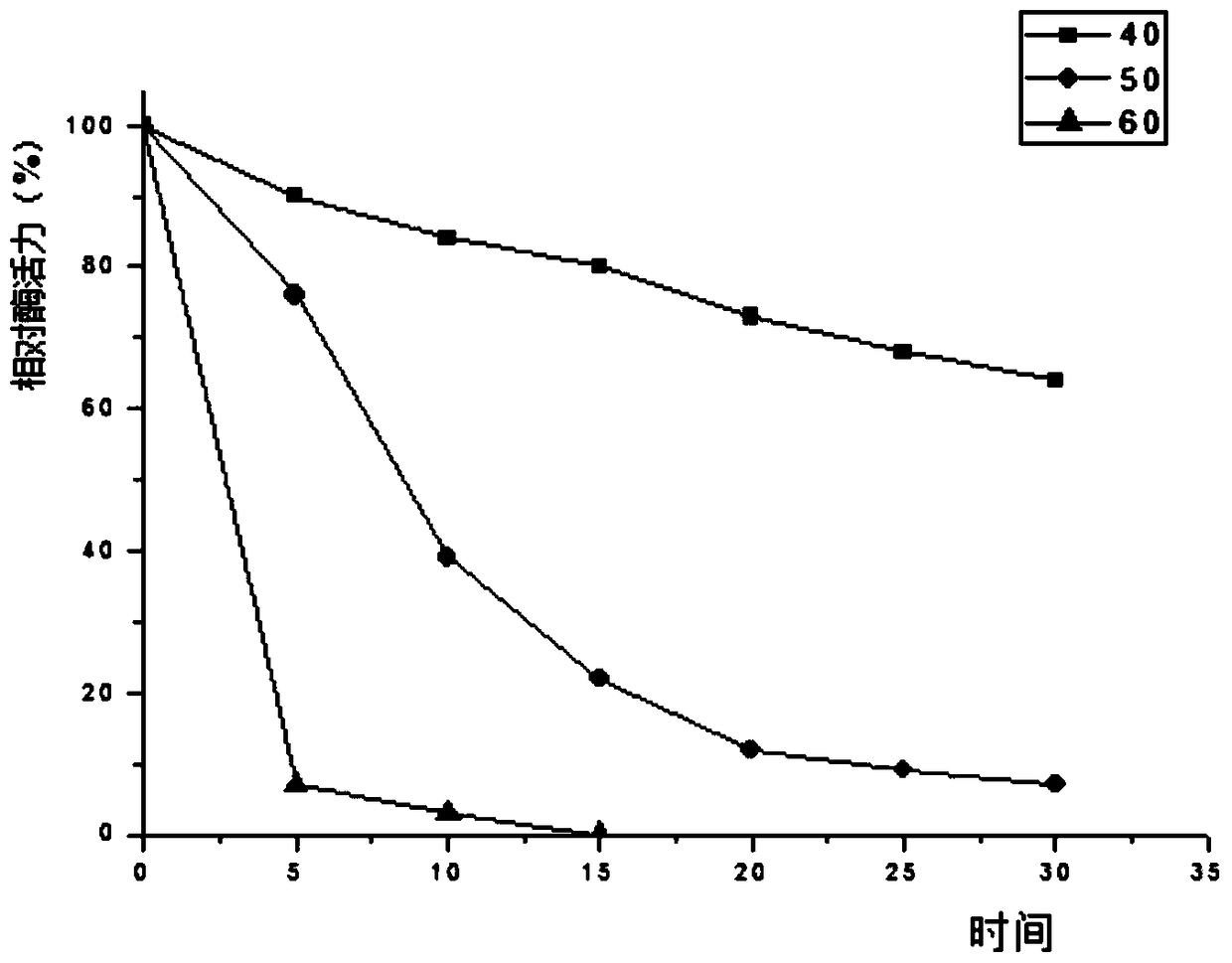
[0088] 3.3 The collected supernatant was first loaded onto a Ni-NTA affinity column with a column volume of 1 mL with 10 mL of NPI solution containing 10 mM imidazole at a rate of 1.0 mL / min. Then use NPI-enzyme solution, NPI solutions containing 20mM, 50mM, and 200mM to elute the affinity column in turn, measure the enzyme activity of the eluate and perform SDS-PAGE electrophoresis to determine the optimal purification conditions and obtain pure GpA1 protein ,Such as figure 1 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com