Bispecific CD3 and CD19 antigen binding constructs
A bispecific, construct technology, applied in the direction of antibodies, anti-receptors/cell surface antigens/cell surface determinants, immunoglobulins, anti-inflammatory agents, etc. Anti-adverse in vivo half-life and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0278] Example 1. Description of bispecific anti-CD19-CD3 antigen binding constructs.
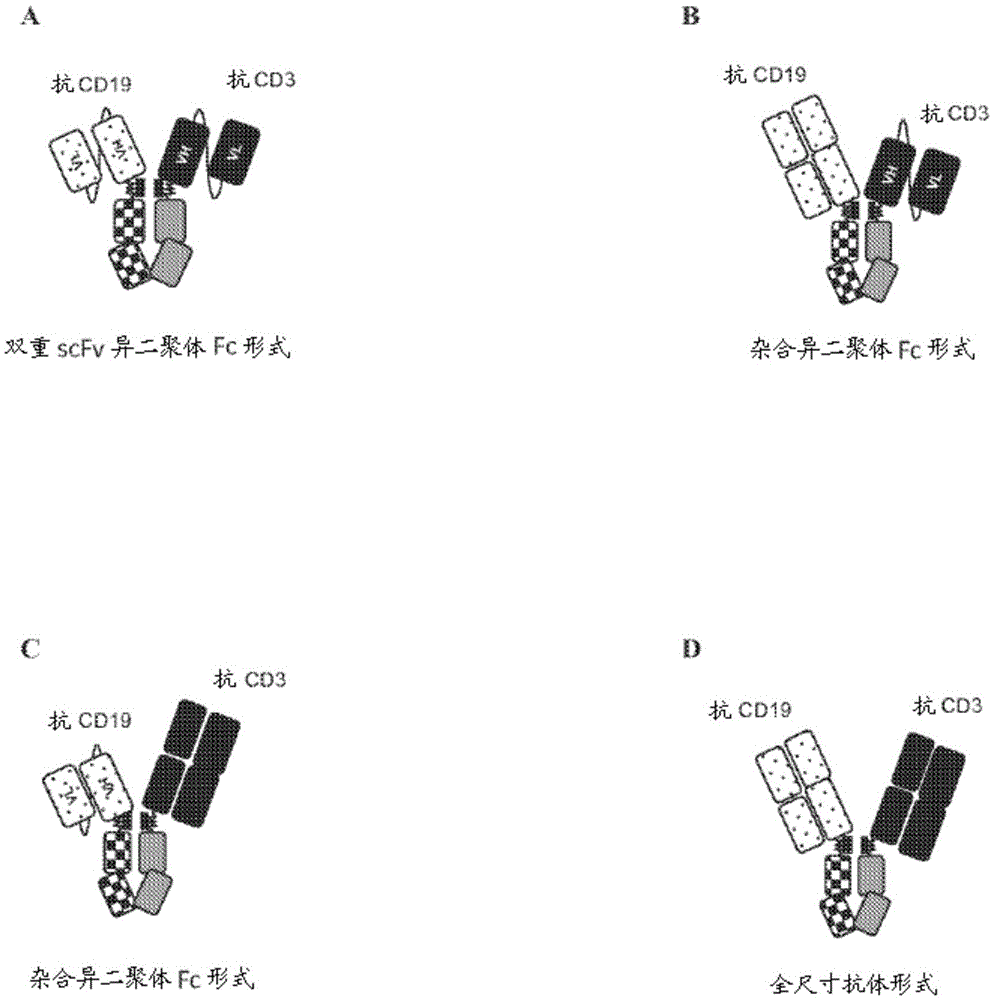
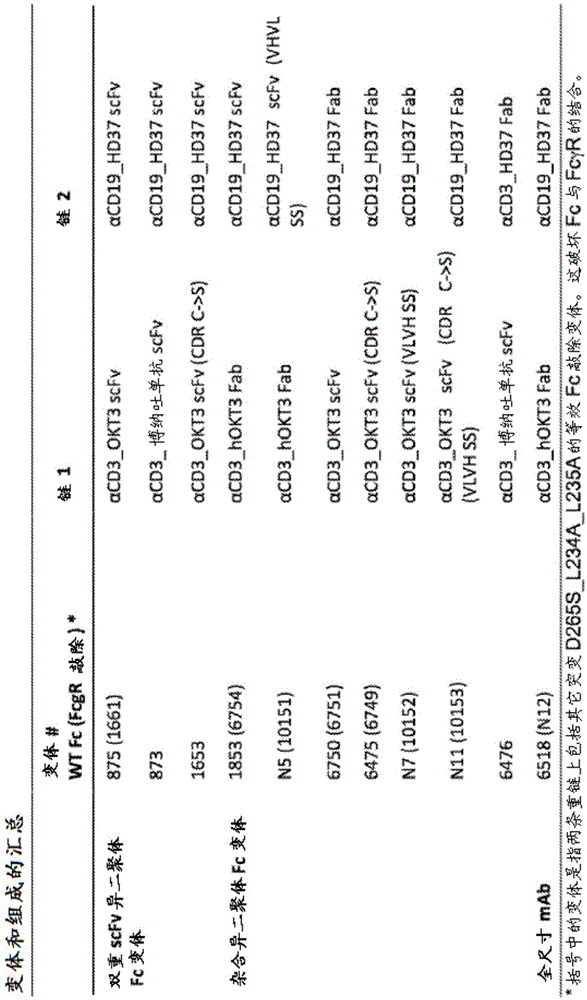
[0279] A number of exemplary bispecific anti-CD3-CD19 antigen binding constructs were designed as described below. figure 1 Exemplary schematics of such constructs are shown in A-C. figure 2 A summary of these variants is shown in . All formats are based on a heterodimeric Fc constructed from known mutations in the CH3 domain (Von Kreudenstein et al., MAbs. 20135(5):646-54):
[0280] The dual scFv heterodimeric Fc molecule contains a heterodimeric Fc with anti-CD19 scFv and anti-CD3 scFv
[0281] • The hybrid heterodimeric Fc molecule comprises a heterodimeric Fc with anti-CD19 scFv and anti-CD3 Fab or a heterodimeric Fc with anti-CD19 Fab and anti-CD3 scFv.
[0282] • A full-size heterodimeric Fc molecule comprising a heterodimeric Fc with anti-CD19 Fab and anti-CD3 Fab; the full-size molecule can be constructed from a common light chain or with an anti-CD19 light chain and an anti-C...
Embodiment 2
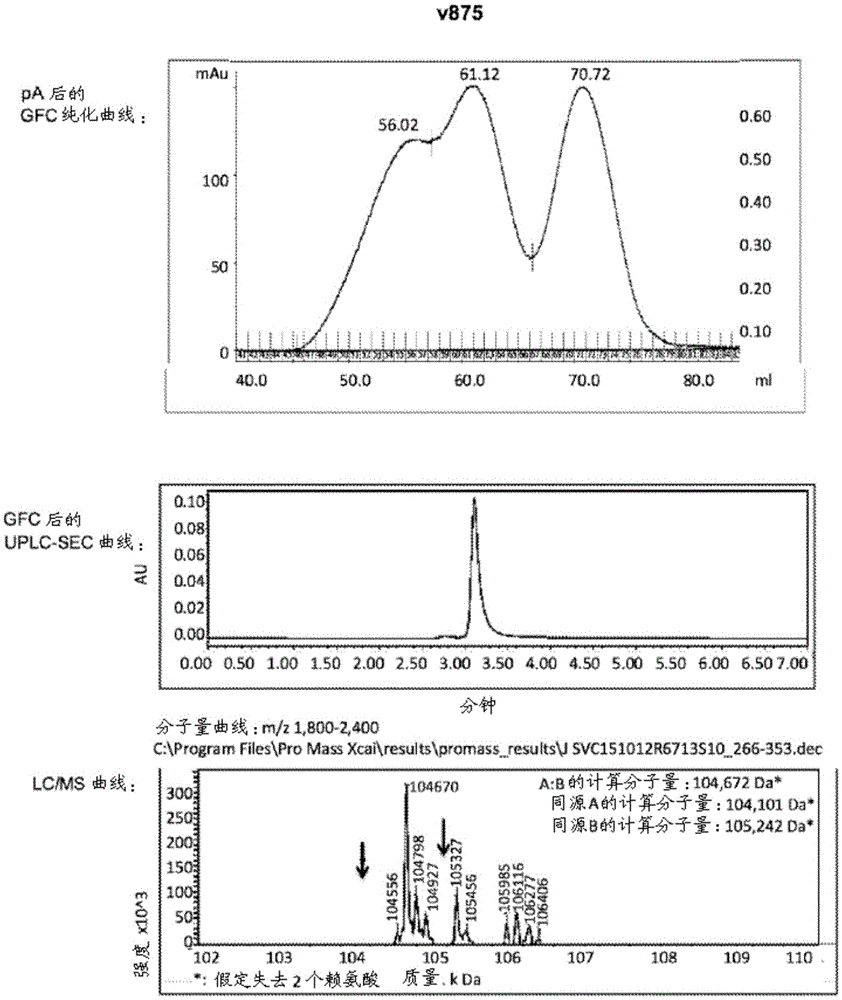
[0297] Example 2: Cloning, Expression and Purification of Exemplary Antigen Binding Constructs
[0298] The variants described in Example 1 (antigen binding constructs) and controls were cloned and expressed as follows. Genes encoding antibody heavy and light chains were constructed via gene synthesis using codons optimized for human / mammalian expression. The scFv and Fab sequences were obtained from the known anti-CD19 antibody HD37 (HD37, Kipriyanov et al., 1998, Int. J Cancer: 77, 763-772) and the known anti-CD3 monoclonal antibody OKT3 (ORTHOCLONEOKT3, DrugBank index: DB00075), tirizumab Monoclonal antibody (MGA031, Eli Lilly), blinatumomab (Amgen, US2011 / 0275787) sequences were generated and constructed as described in Example 1.
[0299] The final gene product was subcloned into mammalian expression vector pTT5 (NRC-BRI, Canada) and expressed in CHO cells (Durocher, Y., Perret, S. & Kamen, A. High-level and high-throughput recombinant protein production by transient t...
Embodiment 3
[0305] Example 3: Exemplary bispecific antigen binding as a hybrid heterodimeric Fc or as a full-size antibody Description, Expression and Purification of Synthetic Constructs (Anti-CD3-CD19 or Anti-CD3-CD20)
[0306] V5850, v5851, v5852, v6325, v1813, v1821 and v1823 illustrate bispecific CD3 / CD19 or CD3 / CD20 hybrid antigen binding constructs. These bispecific hybrid variants consist of a Fab on the A or B chain paired with a scFv-Fc on the alternative polypeptide chain. The A chain of the heterodimeric Fc included the following mutations: T350V_L351Y_F405A_Y407V, and the B chain of the heterodimeric Fc included the following mutations: T350V_T366L_K392L_T394W. V1813, v1821 and v1823 illustrate CD3 / CD20 common light chain antigen binding constructs. The common light chain variant is composed of two different Fabs), each Fab) sharing a light chain on a complementary heterodimeric Fc. The composition of the specific variants is indicated in Table 1.
[0307] As for commo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com