Blue fluorescent compound comprising double fluorescence groups and preparation method and application of blue fluorescent compound
A blue fluorescence, dual chromophore technology, applied in the field of organic electroluminescence display, can solve the problems of reducing the stability of phosphorescent dyes, decreasing the luminous quantum efficiency, increasing the non-radiative transition rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Synthesis of compound B1:
[0026]Dissolve 1.00 g (4.8 mmol) of 9,10-phenanthrenequinone, 0.99 g (4.8 mmol) of 9-anthracenaldehyde, 0.92 ml (5.8 mmol) of 4-tert-butylaniline and 4.57 g (59.3 mmol) of ammonium acetate in 50 ml In glacial acetic acid, reflux at 120°C for 24 hours under nitrogen protection. Cool to room temperature, pour the reaction solution into methanol, filter with suction, and then separate by column chromatography (eluent is dichloromethane / petroleum ether=2:1) to obtain the corresponding compound 2-(9-anthracenyl)-1-( 4-tert-butylphenyl)-1H-phenanthrene[9,10-d]imidazole (B1), yield 53%. Synthesize according to the following equation:
[0027]
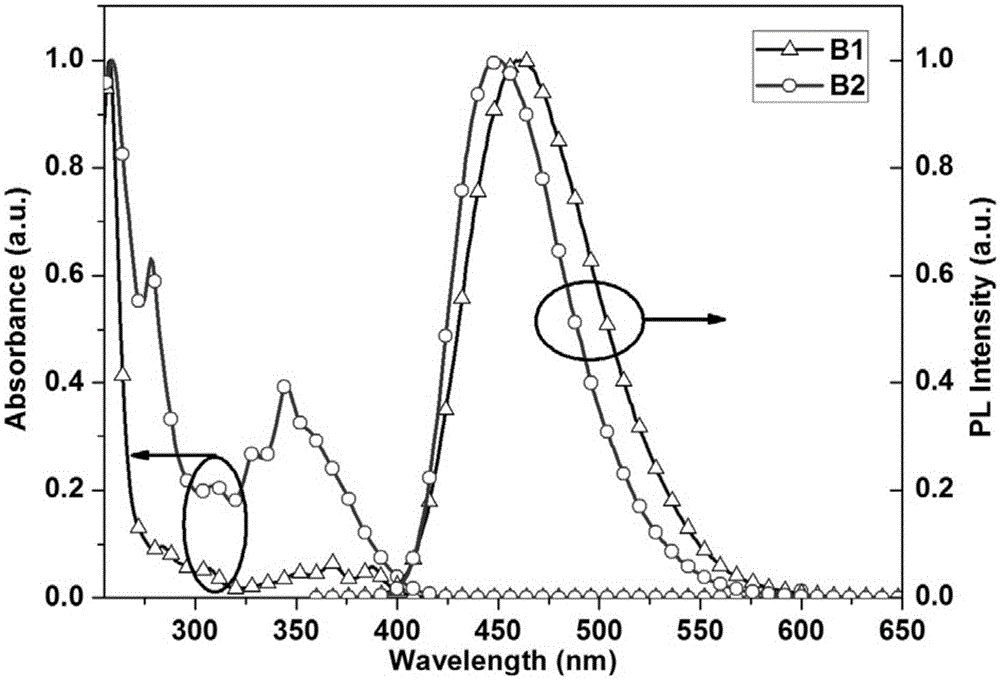
[0028] Compound by 1 HNMR, mass spectrometry, and elemental analysis have been verified, and the results show that the structure is correct, and the data are as follows:
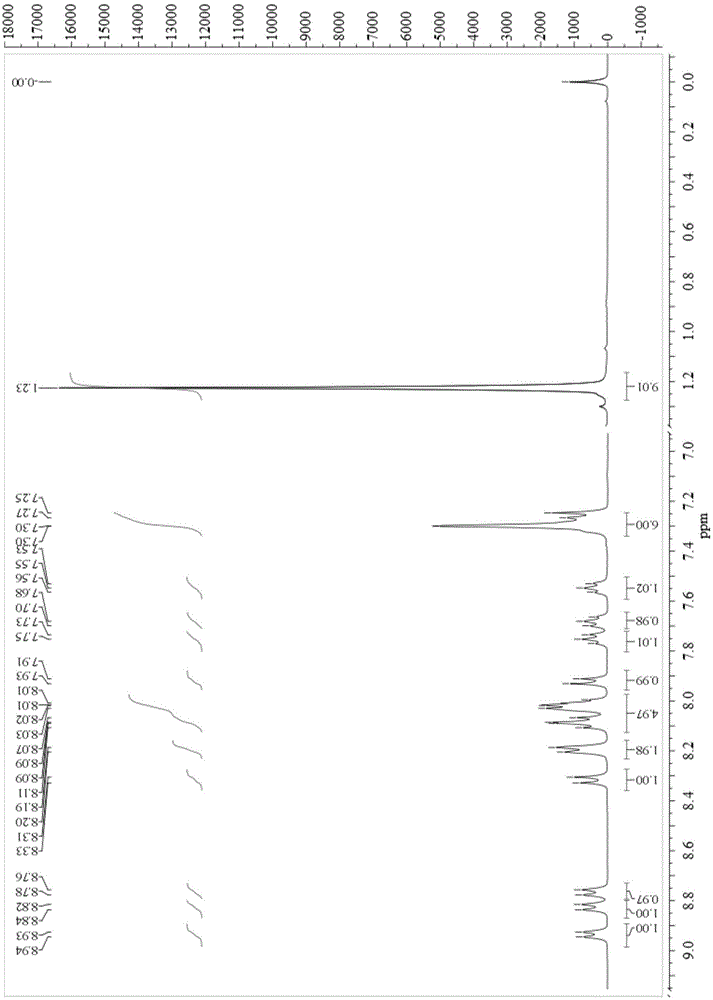
[0029] 1 HNMR (400MHz, CDCl 3 ,ppm):8.91–8.77(m,3H),8.46(s,1H),7.97–7.95(m,2H),7.74–7.67(m,4H),7.55(t,J=8Hzand4Hz,1H),7.41 (t,...
Embodiment 2
[0034] Synthesis of compound B2:
[0035] Dissolve 1.00 g (4.8 mmol) of 9,10-phenanthrenequinone, 1.10 g (4.8 mmol) of 1-pyrene carboxaldehyde, 0.92 ml (5.8 mmol) of 4-tert-butylaniline and 4.57 g (59.3 mmol) of ammonium acetate in 50 ml In glacial acetic acid, reflux at 120°C for 24 hours under nitrogen protection. Cooled to room temperature, the reaction solution was poured into methanol, suction filtered, and then column chromatography (eluent was dichloromethane / petroleum ether=2:1) to obtain the corresponding compound 2-(1-pyrenyl)-1-( 4-tert-butylphenyl)-1H-phenanthrene[9,10-d]imidazole (B2), yield 74%. Synthesize according to the following equation:
[0036]
[0037] Compound by 1 HNMR, mass spectrometry, and elemental analysis have been verified, and the results show that the structure is correct, and the data are as follows:
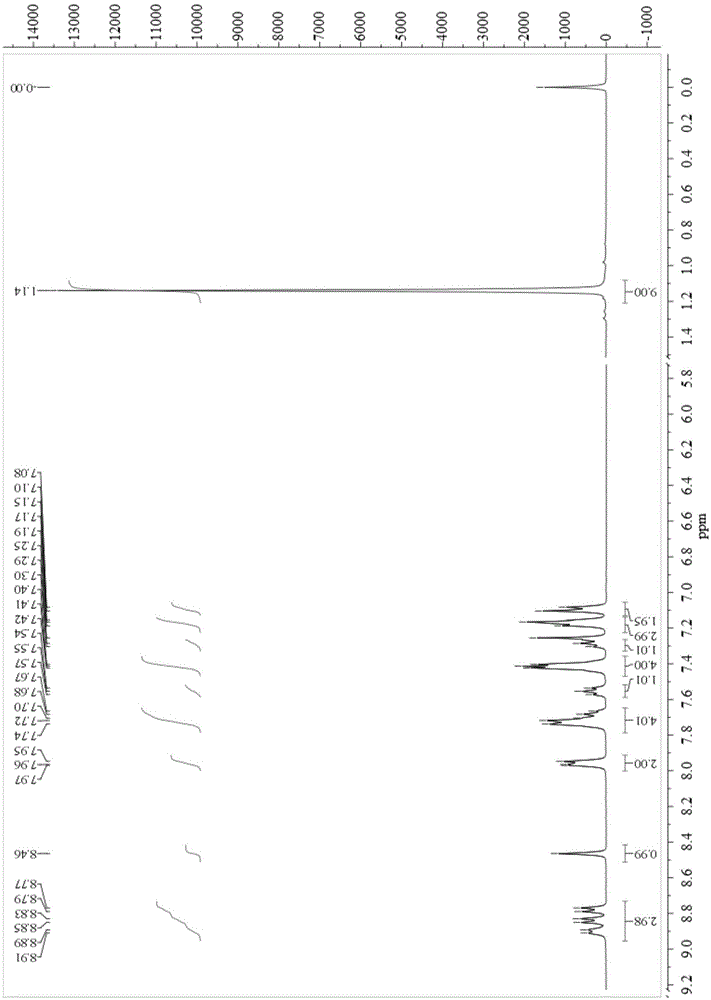
[0038] 1 HNMR (400MHz, CDCl 3 ,ppm):8.94(d,J=8Hz,1H),8.84-8.76(m,2H),8.33(d,J=8Hz,1H),8.20(d,J=4Hz,2H),8.11-8.00( m,5H),7.93(d,J=8H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| external quantum efficiency | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
| internal quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap