A kind of chroman ring derivative and its preparation method
A technology for chroman rings and derivatives, which is applied in the field of chroman ring derivatives and their preparation, can solve problems such as difficult synthesis methods, and achieve broad application prospects and complex and diverse structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
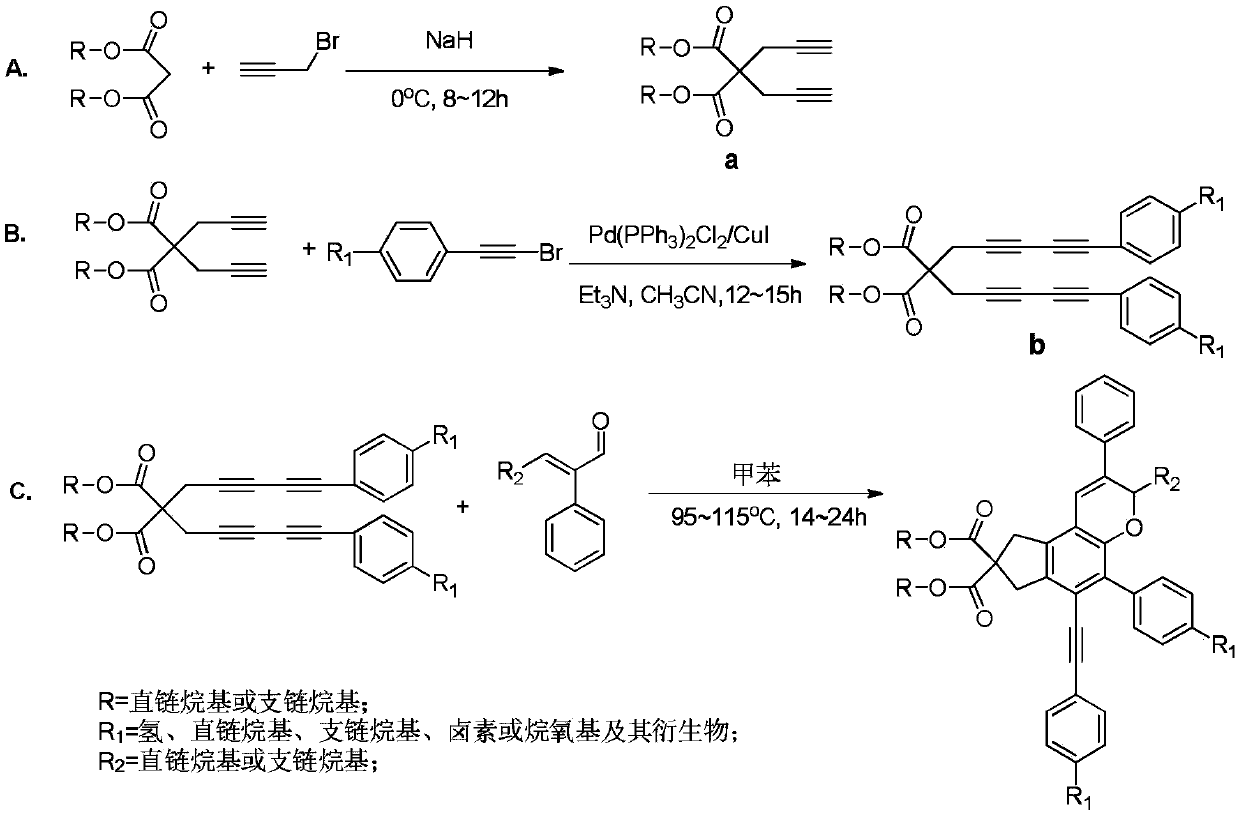
[0036] A chroman ring derivative having the following structural formula:
[0037]
[0038] Its synthesis route is as follows figure 2 shown.
[0039] The preparation method of above-mentioned chroman ring derivative is:
[0040] A. Using 820mmol of sodium hydride as a catalyst, 200mmol of diisopropyl malonate and 440mmol of propargyl bromide were added to 250mL of anhydrous acetonitrile, stirred and reacted in an ice-water bath for 8 hours, the product was washed with water, extracted with ethyl acetate, Spin to dry under reduced pressure, perform column chromatography with ethyl acetate:petroleum ether=1:100 (volume ratio) as the eluent, collect the target product, concentrate and dry to obtain a white solid product, namely compound a-1;
[0041] B. Under anhydrous and oxygen-free conditions, mix 80mmol of compound a-1 with 224mmol of phenylethynyl bromide in 1.5g of Pd(PPh 3 ) 2 Cl 2 / CuI catalytic system, where Pd(PPh 3 ) 2 Cl 2 The ratio of the amount of subst...
Embodiment 2
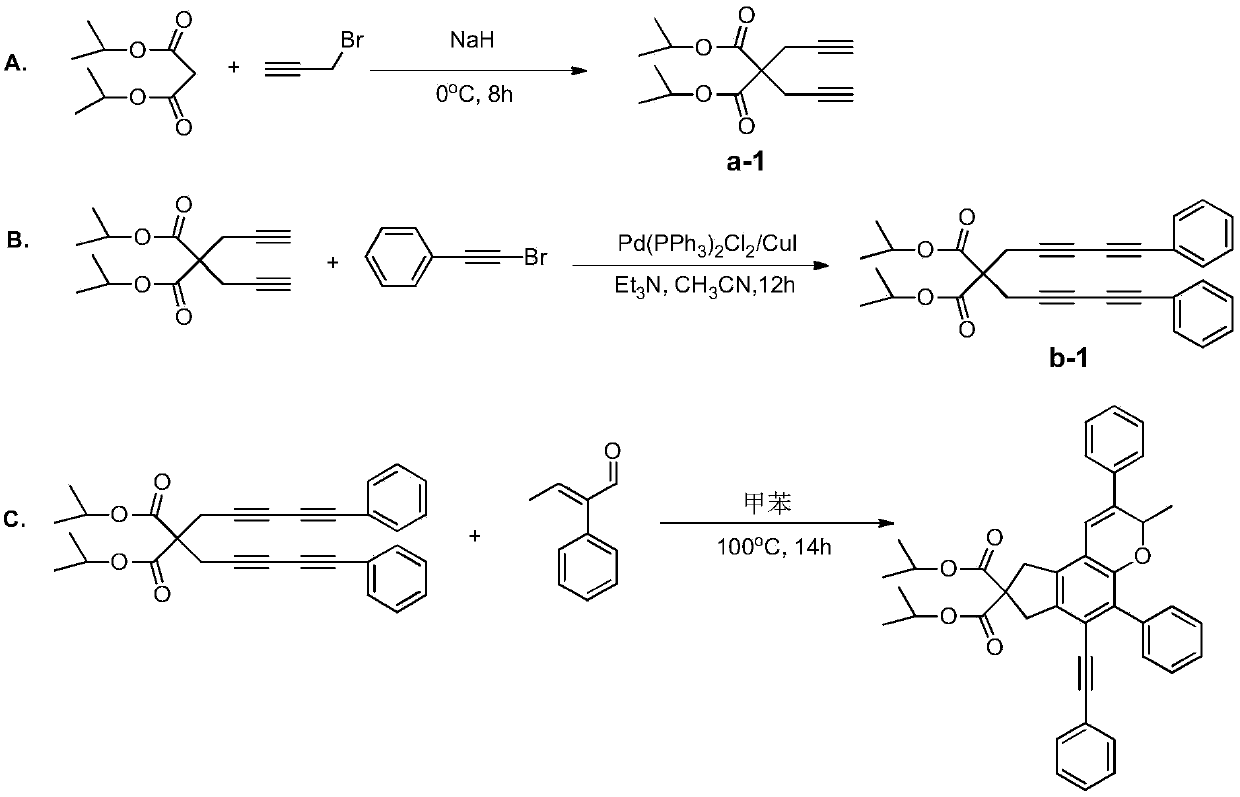
[0047] A chroman ring derivative having the following structural formula:
[0048]
[0049] Its synthesis route is as follows image 3 shown.
[0050] The preparation method of above-mentioned chroman ring derivative is:
[0051] A. With 820mmol sodium hydride as a catalyst, 200mmol dimethyl malonate and 450mmol propargyl bromide were added to 250mL anhydrous acetonitrile, stirred and reacted in an ice-water bath for 8 hours, the product was washed with water, extracted with ethyl acetate, and Press and spin dry, and perform column chromatography with ethyl acetate:petroleum ether=1:100 (volume ratio) as the eluent, collect the target product, concentrate and dry to obtain a white solid product, namely compound a-2.
[0052] B. Under anhydrous and oxygen-free conditions, mix 80mmol of compound a-2 with 200mmol of phenylethynyl bromide substituents in 1.3gPd(PPh 3 ) 2 Cl 2 In / CuI catalytic system, use 340mmol triethylamine as base, 200ml anhydrous acetonitrile as solven...
Embodiment 3
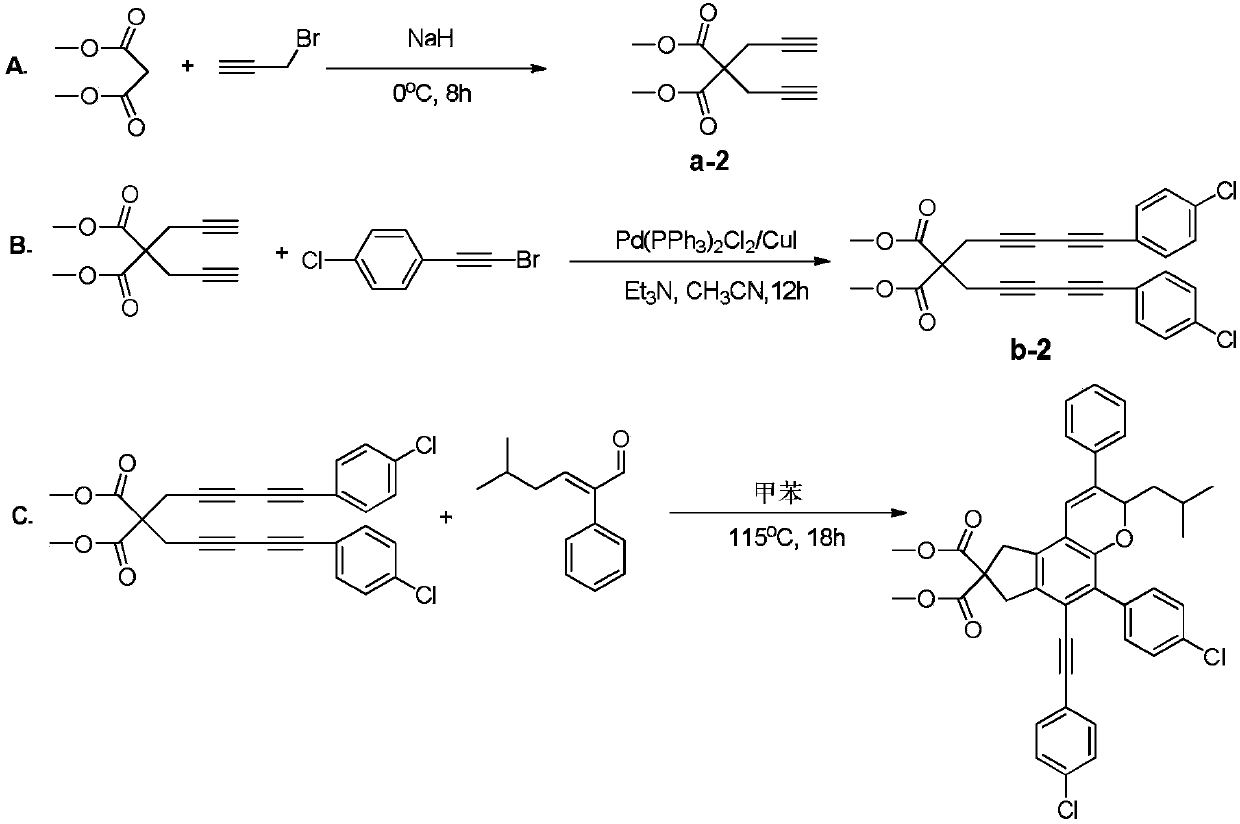
[0059] A chroman ring derivative having the following structural formula:
[0060]
[0061] Its synthesis route is as follows Figure 4 shown.
[0062] The preparation method of above-mentioned chroman ring derivative is:
[0063] A. Using 720mmol sodium hydride as a catalyst, add 200mmol dimethyl malonate and 640mmol propargyl bromide into anhydrous acetonitrile, stir and react in an ice-water bath for 11 hours, add water to wash the product, extract it with ethyl acetate, and depressurize Spin to dry, carry out column chromatography with ethyl acetate:petroleum ether=1:80 (volume ratio) as the eluent, collect the target product, concentrate and dry to obtain a white solid product, namely compound a-3;
[0064] B. Under anhydrous and oxygen-free conditions, mix 80mmol of compound a-3 with 240mmol of phenylethynyl bromide in 1.1g of Pd (PPh 3 ) 2 Cl 2 / CuI catalytic system, the molar ratio Pd(PPh 3 ) 2 Cl 2 :CuI=3:1, with 400mmol triethylamine as the base, 140mL anh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com