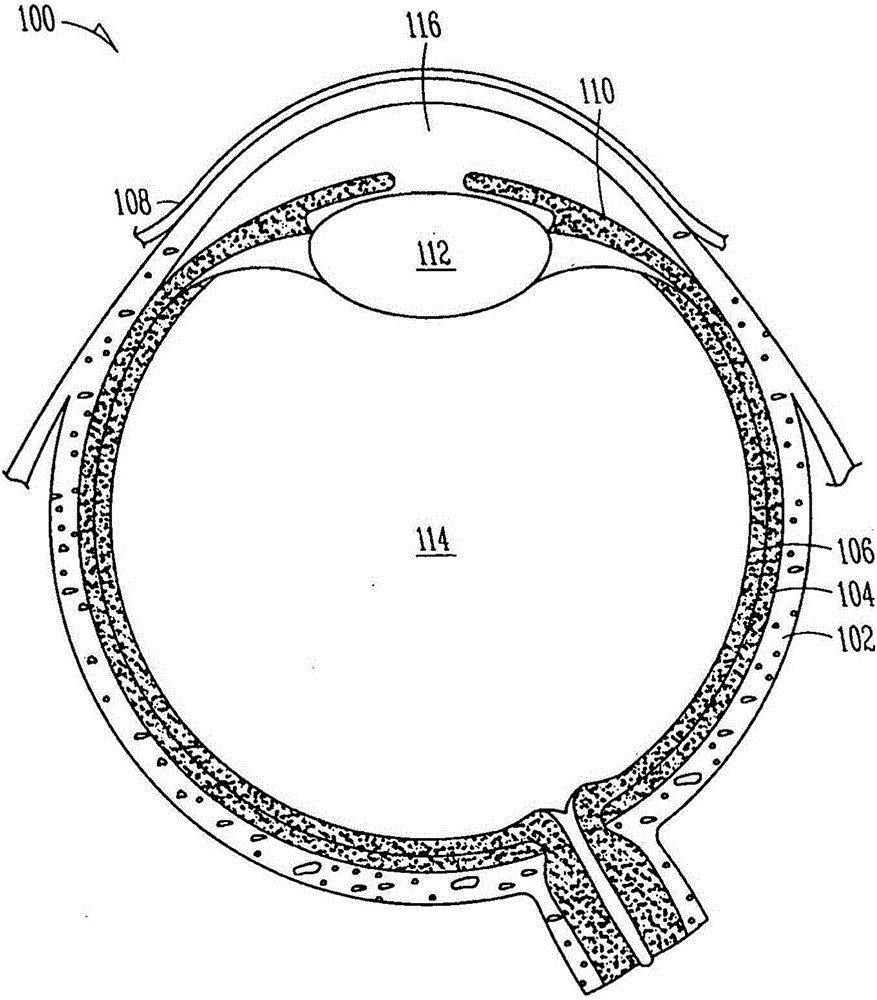
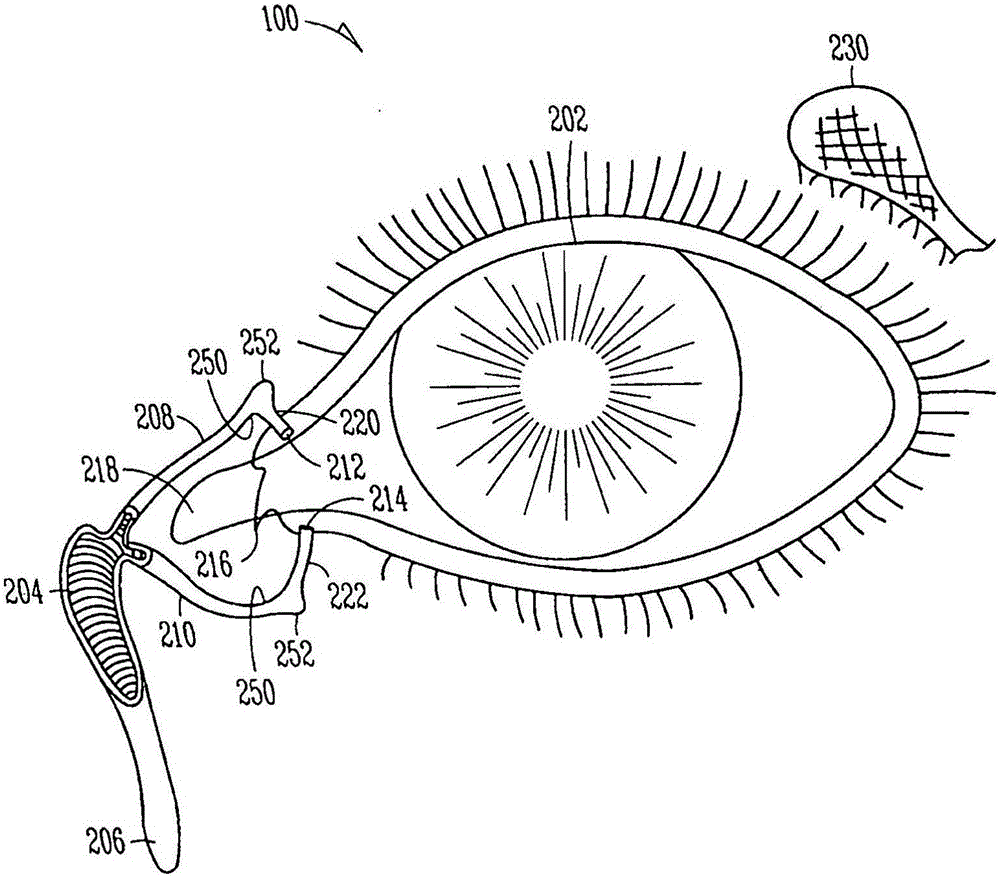
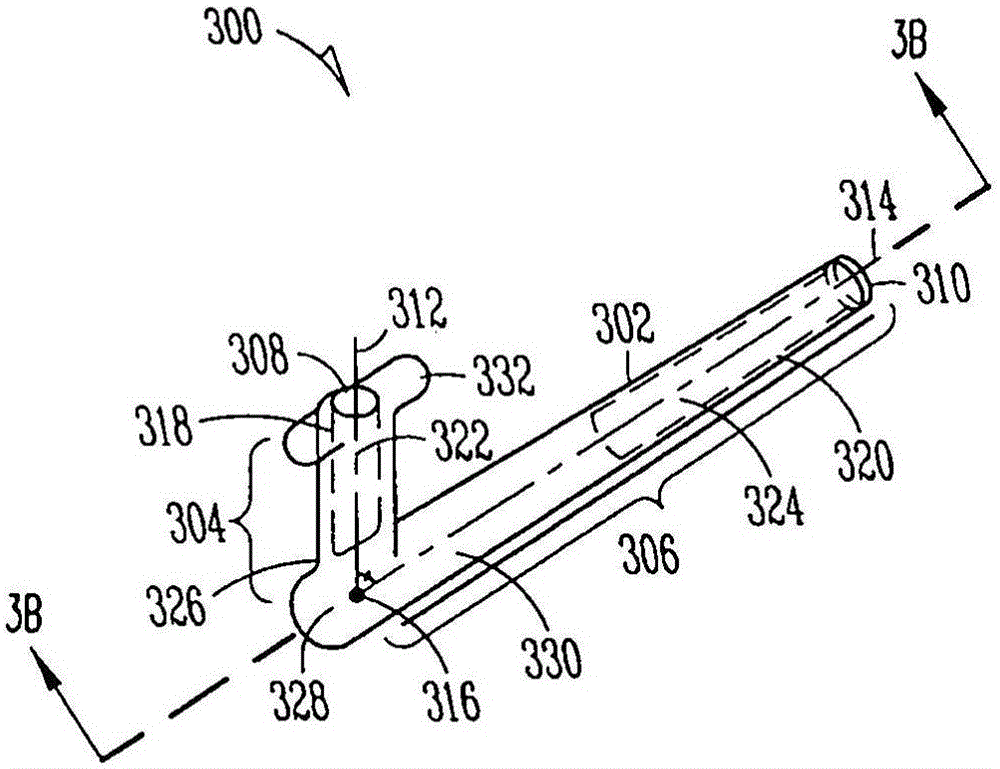
Lacrimal implants
An implant, lacrimal gland technology, applied in the field of lacrimal gland implants, eye implants, can solve problems such as inefficiency, lack of patient compliance or non-localized drug delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0243] Figure 46 A lacrimal implant is shown including a notch extending from the proximal end of the second portion of the implant. In the recess, a swellable hydrogel material is arranged. To allow fluid to be taken up by the hydrogel material, the orifices of the notches are left open.
[0244] At 4602 is indicated the hydrogel material and part of the surrounding implant at 30 minutes of hydration. At 4604 is indicated the hydrogel material and part of the surrounding implant at 120 minutes of hydration. At 4606 is indicated the hydrogel material and part of the surrounding implant at 240 minutes of hydration. At 4608 is indicated the hydrogel material and part of the surrounding implant at 1440 minutes of hydration. As shown, the expansion of the hydrogel material causes the surrounding parts of the implant, especially the second part of the implant, to expand outward, such as to the size and shape of the small tube wall, to securely hold the implant. desired positi...
experiment example 2
[0246] Figure 47 A lacrimal implant is shown including a notch extending from the proximal end of the second portion of the implant. In the recess, a swellable hydrogel material is arranged. To allow fluid to be taken up by the hydrogel material, the orifices of the notches are left open.
[0247]In this example, the hydrogel material is constructed and positioned to, when implanted, expand laterally out of the notch and into the ampullary region 4770 to aid in retention of the lacrimal implant within the lacrimal hall. Lateral expansion of the hydrogel material pushes the cap or other protrusion at the proximal end of the first portion flush with the tear hall surface 4772 as shown.
[0248] Examples of sheaths:
[0249] In various ways, the sheath can comprise appropriate shapes and materials to control the migration of drugs or other therapeutic agents from a dissimilar drug insert or implant that includes an integrated drug or other agent. In some examples, the sheath...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com