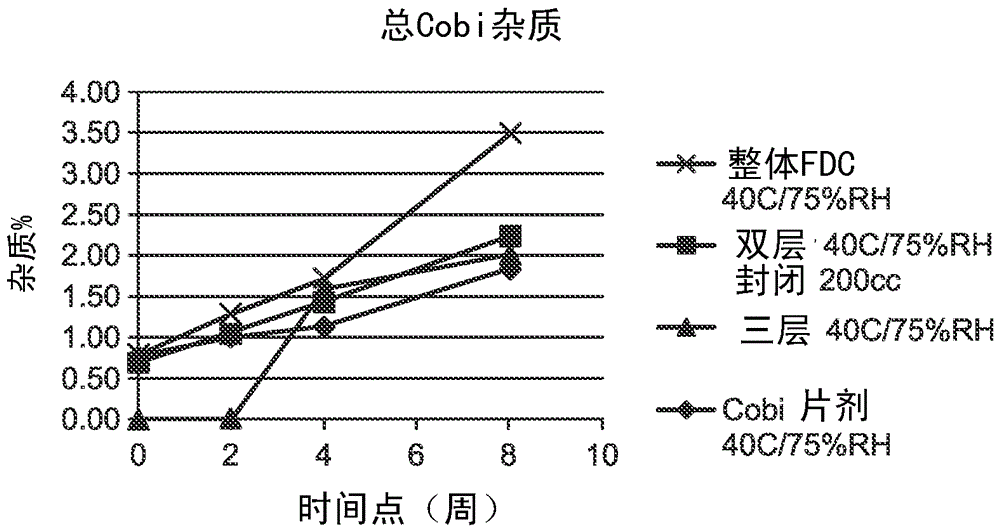
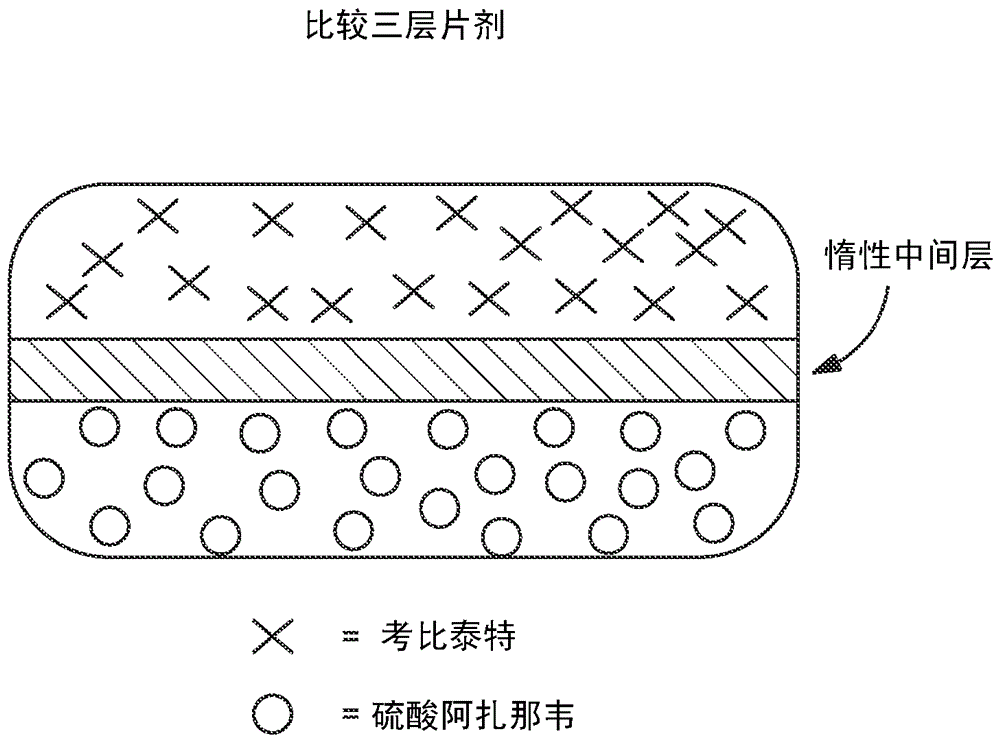
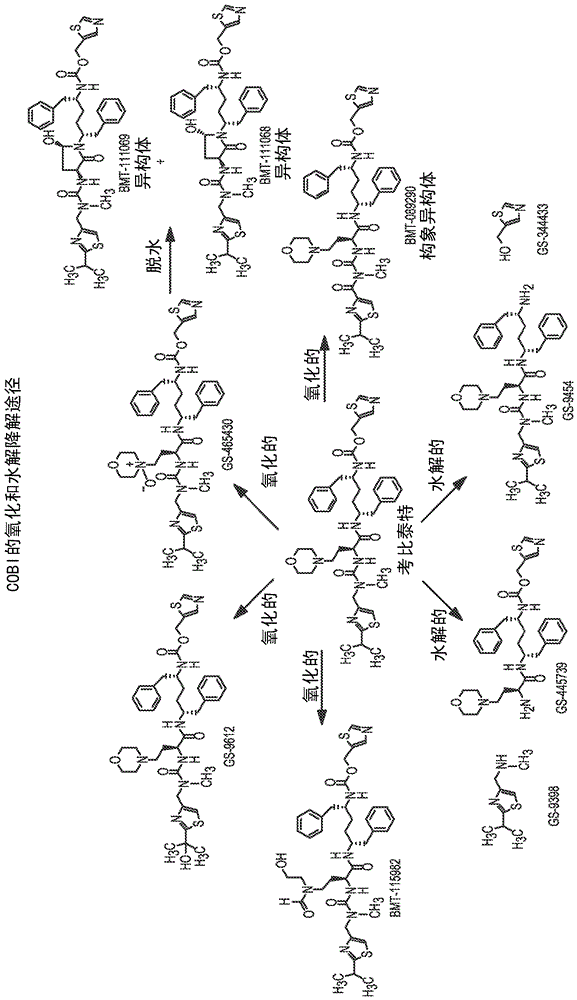
Hiv treatment formulation of atazanavir and cobicistat
一种考比泰特、制剂的技术,应用在阿扎那韦和考比泰特的HIV治疗制剂领域,能够解决给药不方便、患者依从性损害等问题,达到降低丸剂负荷的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 - Manufacturing method
[0054] Manufacture of Atazanavir (ATV) Granules
[0055] Below is a description of the method used to manufacture ATV granulation for ATV / COBI film coated tablets. Materials can be pre-screened when required.
[0056]1. Add microcrystalline cellulose (intragranular part), atazanavir sulfate, stearic acid, sodium starch glycolate (intragranular part), crospovidone (intragranular part) and hydroxypropyl cellulose to a suitable in a mixer, and blend the materials.
[0057] 2. Wet granulate the premix from step 1 with water.
[0058] 3. Wet mill the granulation from step 2 using a suitable Comil.
[0059] 4. Dry the granules from step 3 in a suitable fluid bed drier.
[0060] 5. Size the dry pellet from step 4 using the appropriate Comil.
[0061] 6. Add microcrystalline cellulose (extragranular fraction), sodium starch glycolate (extragranular fraction), and crospovidone (extragranular fraction) to the granules from step 5 and ble...
Embodiment 2
[0076] Example 2 Batch size and formulation
[0077] Representative batch sizes and formulations for ATV / COBI film-coated tablets are provided in Tables 1 and 2, respectively.
[0078]
[0079]
[0080]
[0081] NOTE: The amount of atazanavir (as free base) is theoretically equivalent to 87.8% atazanavir sulfate.
[0082] The amount shown is equivalent to 150 mg corbytide (without silicon dioxide). The amount of corbitide-on-silica was adjusted based on the drug content factor (DCF) for corbitide-on-silica, with a concomitant adjustment in microcrystalline cellulose.
[0083] q.s. = adequate; NA = not applicable.
Embodiment 3
[0084] Example 3 - The composition of an ATV / COBI film-coated tablet with 300 mg (as free base) of atazanavir sulfate / 150 mg of corbitide is set forth in Table 3 below:
[0085]
[0086] The amount of atazanavir (as free base) is theoretically equivalent to 87.8% atazanavir sulfate. The amount is 87.8% based on a theoretical determination "as is"; 341.7 mg of atazanavir sulfate is equivalent to 300 mg of atazanavir. Adjustments may be made "as is" based on actual assays of the drug lot batches used in the manufacture of the drug product.
[0087] Corbytate Pharmaceuticals is Corbytate on silica. The amount shown is equivalent to 150 mg corbytide (without silicon dioxide). The amount of corbitide-on-silica was adjusted based on the drug content factor (DCF) for corbitide-on-silica, with a concomitant adjustment in microcrystalline cellulose.
[0088] (NC = Non-Compendial NF = National Formulary Ph.Eur. = European Pharmacopoeia USP = United States Pharmacopoeia).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com