Cobicistat raw material impurity preparation method
A technology of comparability and impurities, applied in the field of medicine, can solve problems such as unqualified quality of finished products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
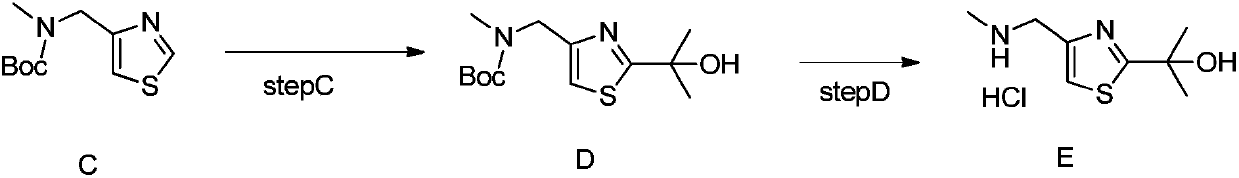
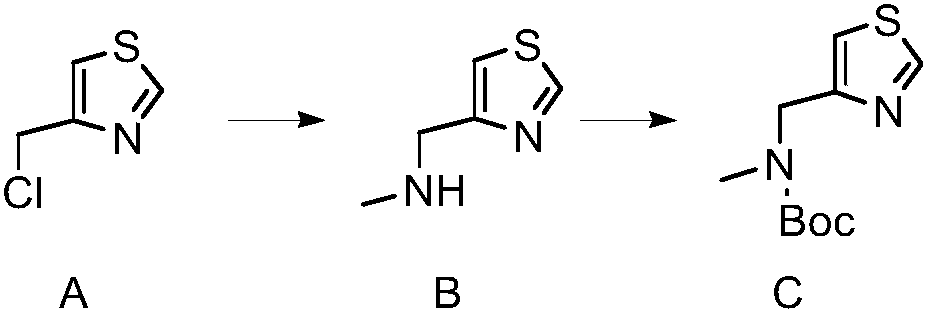
[0033] Add 8.5g of boc-protected 4-ethylaminomethyl-thiazole and 100ml of tetrahydrofuran into a 1L three-necked flask, add 2ml of 2.5M n-butyllithium at -78°C, stir for 30 minutes, add 2ml of acetone, stir for 30 minutes and then Add 10ml of 10% ammonium chloride solution at room temperature, add 500ml ethyl acetate to wash and separate the liquid, and concentrate the ethyl acetate phase to dryness to obtain 5g of 2-isopropyl-4-(methyl-boc aminomethyl)thiazole, M+ H=287, purity 96%.
Embodiment 2
[0035] Add 3g of 2-isopropyl-4-(methyl-bocaminomethyl)thiazole, 10ml of trifluoroacetic acid, and 50ml of ethanol into a 1L three-necked flask, and react at 40°C for 5 hours to obtain 1g of hydroxythiazole, M+H =187, purity 98%.
Embodiment 3
[0037] When the impurity E is 1.0%, the moisture content of bisestat is 0.55% after 24 hours of drying time; when the impurity derivative is 0.15%, the moisture content of bisestat is 0.10% after 3 hours of drying time.
[0038] Example: 4:
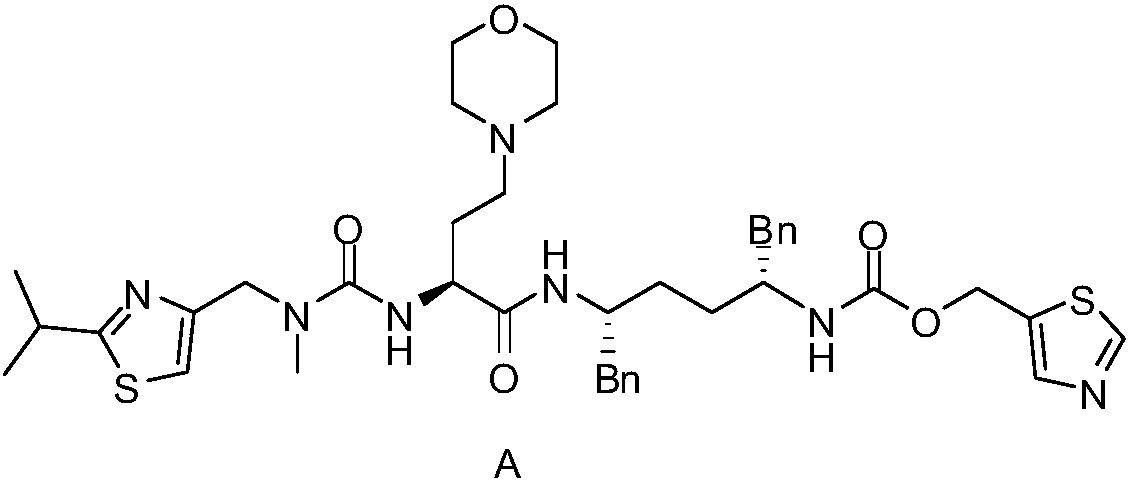
[0039] When the impurity E contains 0.5%, after multi-step conversion, it still exists in the cobicistat API in the form of 0.4% structure as shown in the following formula structure F, M+H=792, and the structure is as follows:
[0040]
[0041] This impurity is very similar to the API structure of cobicistat and is difficult to purify.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com